medical device or a medicinal product
medical device or a medicinal product
medical device or a medicinal product
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
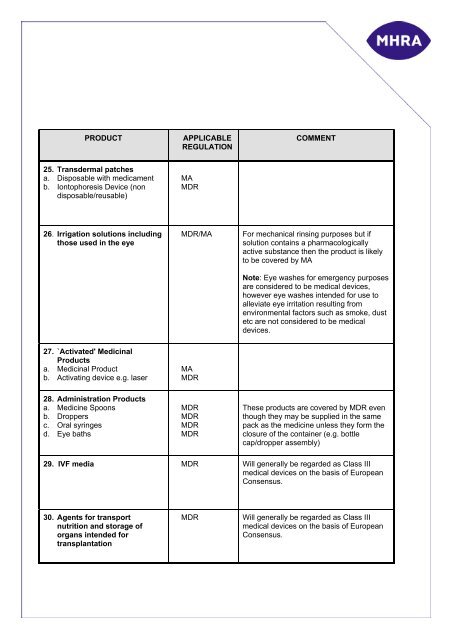
PRODUCT<br />
APPLICABLE<br />
REGULATION<br />
COMMENT<br />
25. Transdermal patches<br />
a. Disposable with medicament<br />
b. Iontoph<strong>or</strong>esis Device (non<br />
disposable/reusable)<br />
MA<br />
MDR<br />
26. Irrigation solutions including<br />
those used in the eye<br />
MDR/MA<br />
F<strong>or</strong> mechanical rinsing purposes but if<br />
solution contains a pharmacologically<br />
active substance then the <strong>product</strong> is likely<br />
to be covered by MA<br />
Note: Eye washes f<strong>or</strong> emergency purposes<br />
are considered to be <strong>medical</strong> <strong>device</strong>s,<br />
however eye washes intended f<strong>or</strong> use to<br />
alleviate eye irritation resulting from<br />
environmental fact<strong>or</strong>s such as smoke, dust<br />
etc are not considered to be <strong>medical</strong><br />
<strong>device</strong>s.<br />
27. `Activated' Medicinal<br />
Products<br />
a. Medicinal Product<br />
b. Activating <strong>device</strong> e.g. laser<br />
MA<br />
MDR<br />
28. Administration Products<br />
a. Medicine Spoons<br />
b. Droppers<br />
c. Oral syringes<br />
d. Eye baths<br />
MDR<br />
MDR<br />
MDR<br />
MDR<br />
These <strong>product</strong>s are covered by MDR even<br />
though they may be supplied in the same<br />
pack as the medicine unless they f<strong>or</strong>m the<br />
closure of the container (e.g. bottle<br />
cap/dropper assembly)<br />
29. IVF media MDR Will generally be regarded as Class III<br />
<strong>medical</strong> <strong>device</strong>s on the basis of European<br />
Consensus.<br />
30. Agents f<strong>or</strong> transp<strong>or</strong>t<br />
nutrition and st<strong>or</strong>age of<br />
<strong>or</strong>gans intended f<strong>or</strong><br />
transplantation<br />
MDR<br />
Will generally be regarded as Class III<br />
<strong>medical</strong> <strong>device</strong>s on the basis of European<br />
Consensus.