medical device or a medicinal product
medical device or a medicinal product
medical device or a medicinal product
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
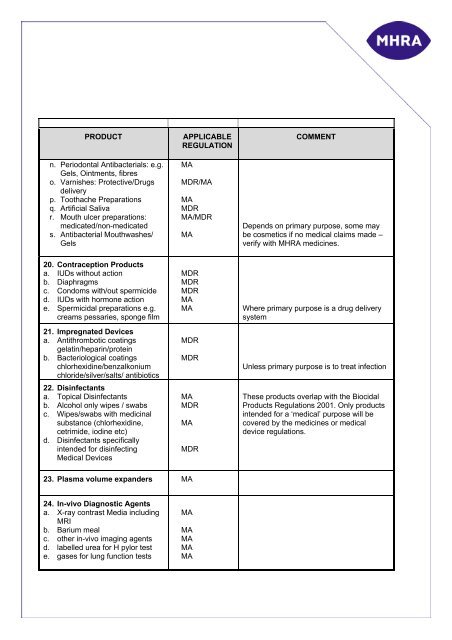
PRODUCT<br />
n. Periodontal Antibacterials: e.g.<br />
Gels, Ointments, fibres<br />
o. Varnishes: Protective/Drugs<br />
delivery<br />
p. Toothache Preparations<br />
q. Artificial Saliva<br />
r. Mouth ulcer preparations:<br />
medicated/non-medicated<br />
s. Antibacterial Mouthwashes/<br />
Gels<br />
APPLICABLE<br />
REGULATION<br />
MA<br />
MDR/MA<br />
MA<br />
MDR<br />
MA/MDR<br />
MA<br />
COMMENT<br />
Depends on primary purpose, some may<br />
be cosmetics if no <strong>medical</strong> claims made –<br />
verify with MHRA medicines.<br />
20. Contraception Products<br />
a. IUDs without action<br />
b. Diaphragms<br />
c. Condoms with/out spermicide<br />
d. IUDs with h<strong>or</strong>mone action<br />
e. Spermicidal preparations e.g.<br />
creams pessaries, sponge film<br />
21. Impregnated Devices<br />
a. Antithrombotic coatings<br />
gelatin/heparin/protein<br />
b. Bacteriological coatings<br />
chl<strong>or</strong>hexidine/benzalkonium<br />
chl<strong>or</strong>ide/silver/salts/ antibiotics<br />
22. Disinfectants<br />
a. Topical Disinfectants<br />
b. Alcohol only wipes / swabs<br />
c. Wipes/swabs with <strong>medicinal</strong><br />
substance (chl<strong>or</strong>hexidine,<br />
cetrimide, iodine etc)<br />
d. Disinfectants specifically<br />
intended f<strong>or</strong> disinfecting<br />
Medical Devices<br />
MDR<br />
MDR<br />
MDR<br />
MA<br />
MA<br />
MDR<br />
MDR<br />
MA<br />
MDR<br />
MA<br />
MDR<br />
Where primary purpose is a drug delivery<br />
system<br />
Unless primary purpose is to treat infection<br />
These <strong>product</strong>s overlap with the Biocidal<br />
Products Regulations 2001. Only <strong>product</strong>s<br />
intended f<strong>or</strong> a ‘<strong>medical</strong>’ purpose will be<br />
covered by the medicines <strong>or</strong> <strong>medical</strong><br />
<strong>device</strong> regulations.<br />
23. Plasma volume expanders MA<br />
24. In-vivo Diagnostic Agents<br />
a. X-ray contrast Media including<br />
MRI<br />
b. Barium meal<br />
c. other in-vivo imaging agents<br />
d. labelled urea f<strong>or</strong> H pyl<strong>or</strong> test<br />
e. gases f<strong>or</strong> lung function tests<br />
MA<br />
MA<br />
MA<br />
MA<br />
MA