PIONEER JUNIOR COLLEGE - ASKnLearn
PIONEER JUNIOR COLLEGE - ASKnLearn
PIONEER JUNIOR COLLEGE - ASKnLearn
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2<br />
5 Which of the following statements best explains why ammonia deviates from ideal<br />
gas behaviour?<br />
A<br />
B<br />
C<br />
D<br />
The bonds between atoms in ammonia molecules are strong.<br />
Ammonia can be easily compressed at low temperature and high pressure.<br />
There are strong intermolecular forces of attractions between ammonia<br />
molecules.<br />
The volume occupied by ammonia molecules is significant compared to the<br />
volume occupied by the gas.<br />
6 Iodine trichloride, ICl 3 , is made by reacting iodine with chlorine.<br />
I 2 (s) + Cl 2 (g) → 2ICl(s) ΔH Ө = +14 kJ mol -1<br />
ICl(s) + Cl 2 (g) → ICl 3 (s) ΔH Ө = -88 kJ mol -1<br />
Given the data above, what is the standard enthalpy change of formation for solid<br />
iodine trichloride?<br />
A -60 kJ mol -1<br />
B -74 kJ mol -1<br />
C -81 kJ mol -1<br />
D -162 kJ mol -1<br />
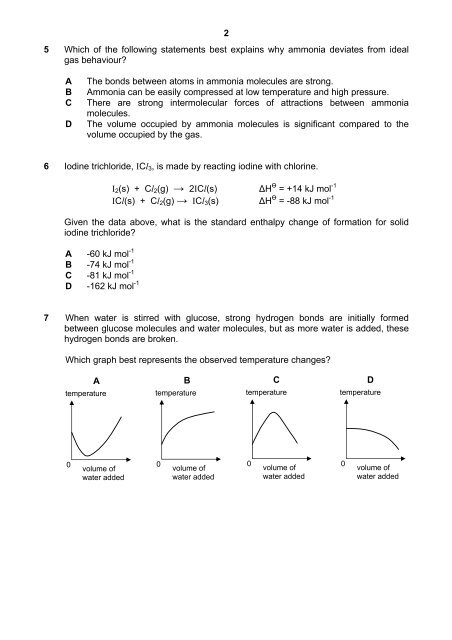
7 When water is stirred with glucose, strong hydrogen bonds are initially formed<br />
between glucose molecules and water molecules, but as more water is added, these<br />
hydrogen bonds are broken.<br />
Which graph best represents the observed temperature changes?<br />
A<br />
temperature<br />
B<br />
temperature<br />
C<br />
temperature<br />
D<br />
temperature<br />
0<br />
volume of<br />
water added<br />
0<br />
volume of<br />
water added<br />
0<br />
volume of<br />
water added<br />
0<br />
volume of<br />
water added