PIONEER JUNIOR COLLEGE - ASKnLearn
PIONEER JUNIOR COLLEGE - ASKnLearn
PIONEER JUNIOR COLLEGE - ASKnLearn
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
4<br />
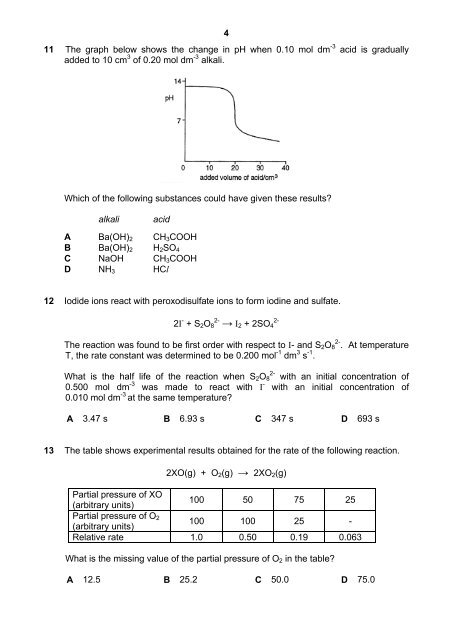
11 The graph below shows the change in pH when 0.10 mol dm -3 acid is gradually<br />
added to 10 cm 3 of 0.20 mol dm -3 alkali.<br />
Which of the following substances could have given these results?<br />
alkali<br />
acid<br />
A Ba(OH) 2 CH 3 COOH<br />
B Ba(OH) 2 H 2 SO 4<br />
C NaOH CH 3 COOH<br />
D NH 3 HCl<br />
12 Iodide ions react with peroxodisulfate ions to form iodine and sulfate.<br />
2I - + S 2 O 8<br />
2-<br />
→ I 2 + 2SO 4<br />
2-<br />
The reaction was found to be first order with respect to I- and S 2 O 8 2- . At temperature<br />
T, the rate constant was determined to be 0.200 mol -1 dm 3 s -1 .<br />
2-<br />
What is the half life of the reaction when S 2 O 8 with an initial concentration of<br />
0.500 mol dm -3 was made to react with I - with an initial concentration of<br />
0.010 mol dm -3 at the same temperature?<br />
A 3.47 s B 6.93 s C 347 s D 693 s<br />
13 The table shows experimental results obtained for the rate of the following reaction.<br />
2XO(g) + O 2 (g) → 2XO 2 (g)<br />
Partial pressure of XO<br />
(arbitrary units)<br />
100 50 75 25<br />
Partial pressure of O 2<br />
(arbitrary units)<br />
100 100 25 -<br />
Relative rate 1.0 0.50 0.19 0.063<br />
What is the missing value of the partial pressure of O 2 in the table?<br />
A 12.5 B 25.2 C 50.0 D 75.0