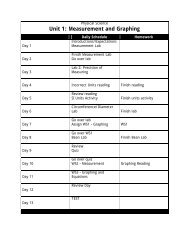
liters solution Molarity = moles solute Another way to express ...
liters solution Molarity = moles solute Another way to express ...
liters solution Molarity = moles solute Another way to express ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
8th hour<br />
Tuesday, May 08, 2012<br />
1:45 PM<br />
<strong>Molarity</strong> = <strong>moles</strong> <strong>solute</strong><br />
<strong>liters</strong> <strong>solution</strong><br />
<strong>Another</strong> <strong>way</strong> <strong>to</strong> <strong>express</strong> concentration (relationship between <strong>solute</strong><br />
and <strong>solution</strong>)<br />
We represent <strong>Molarity</strong> as a unit with a "M" which means "mol<br />
<strong>solute</strong> per L <strong>solution</strong>"<br />
We read M as "molar" out loud. For example 6.0 M KCl… we say:<br />
"6.0 molar KCl" = 6.0 <strong>moles</strong> KCl in 1 liter <strong>solution</strong> = 6.0 <strong>moles</strong> KCl<br />
1 liter <strong>solution</strong><br />
<strong>Molarity</strong> Page 4