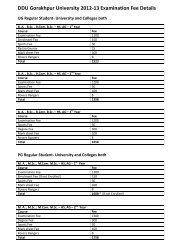
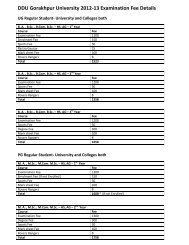
PG Syllabus
PG Syllabus
PG Syllabus
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2. Simple preparation involving two stages. Emphasis should be laid on the following processes :<br />
Purification of the organic compounds, distillation under reduced pressure, steam distillation and<br />
fractional crystallization.<br />
3. Determination of equivalent weight of an acid by direct titration method.<br />
4. Determination of saponification and iodine number of an oil.<br />
Section C (Physical Chemistry)<br />
1. Relative strength of acids by acid hydrolysis of ester.<br />
2. Kinetics of alkali hydrolysis of ester.<br />
3. Partition coefficient of I 2 in two immiscible solvents.<br />
4. Solubility curve of water-acetic acid-chloroform system.<br />
5. Adsorption isotherm of acetic acid on activated charcoal.<br />
6. Adsorption isotherm of oxalic acid on activated charcoal.<br />
7. Determination of heat of solution of a salt by solubility method.<br />
8. Conductometric titration.<br />
9. Preparation of Buffer solution and measurement of pH by pH meter.<br />
10. Setting time of cement in presence of salts with the help of Vicat apparatus.<br />
Distribution of marks<br />
Section A (Inorganic Chemistry)<br />
Exercise 1 21<br />
Exercise 2 14+10<br />
Exercise 3 or 4 5<br />
Section B (Organic Chemistry) 30<br />
Section C (Physical Chemistry) 30<br />
Viva 30<br />
Record 10<br />
------------------------------------------------<br />
Total 150<br />
-------------------------------------------------