Slides
Slides
Slides
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
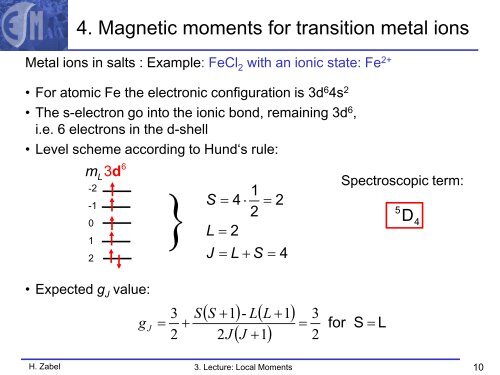
4. Magnetic moments for transition metal ions<br />
Metal ions in salts : Example: FeCl 2 with an ionic state: Fe 2+<br />
• For atomic Fe the electronic configuration is 3d 6 4s 2<br />
• The s-electron go into the ionic bond, remaining 3d 6 ,<br />
i.e. 6 electrons in the d-shell<br />
• Level scheme according to Hund‘s rule:<br />
mL3d<br />
6<br />
-2<br />
}<br />
1<br />
-1<br />
S = 4 ⋅ = 2<br />
2<br />
0<br />
L = 2<br />
1<br />
J = L + S = 4<br />
2<br />
Spectroscopic term:<br />
5 D4<br />
• Expected g J value:<br />
g J<br />
=<br />
3<br />
2<br />
+<br />
S<br />
( S + 1) - L( L + 1)<br />
2J<br />
( J + 1)<br />
=<br />
3<br />
2<br />
for S =<br />
L<br />
H. Zabel 3. Lecture: Local Moments<br />
10