Tunneling
Tunneling
Tunneling
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
5<br />
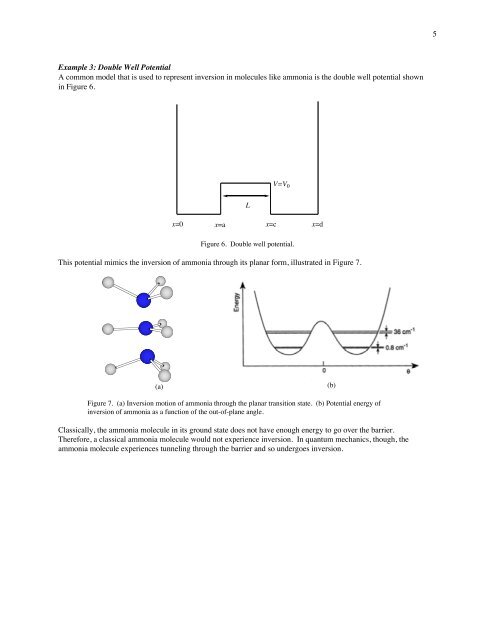
Example 3: Double Well Potential<br />
A common model that is used to represent inversion in molecules like ammonia is the double well potential shown<br />
in Figure 6.<br />
V=V 0<br />
L<br />
x=0<br />
x=a x=c x=d<br />
Figure 6. Double well potential.<br />
This potential mimics the inversion of ammonia through its planar form, illustrated in Figure 7.<br />
(a)<br />
(b)<br />
Figure 7. (a) Inversion motion of ammonia through the planar transition state. (b) Potential energy of<br />
inversion of ammonia as a function of the out-of-plane angle.<br />
Classically, the ammonia molecule in its ground state does not have enough energy to go over the barrier.<br />
Therefore, a classical ammonia molecule would not experience inversion. In quantum mechanics, though, the<br />
ammonia molecule experiences tunneling through the barrier and so undergoes inversion.