Heat transfer â lecture 1
Heat transfer â lecture 1
Heat transfer â lecture 1
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Heat</strong> <strong>transfer</strong> – <strong>lecture</strong> 1<br />
LITERATURE<br />
Bejan A., Kraus A.D.: <strong>Heat</strong> Transfer Handbook. John Wiley & Sons, Inc., New<br />
Jersey 2003<br />
Baehr H.D., Stephan K.: <strong>Heat</strong> and Mass Transfer. Springer, Berlin 2006<br />
NOMENCLATURE<br />
Roman Letter Symbols<br />
A, (F, S) cross-sectional area, m 2<br />
c specific heat, W/m · K<br />
d differential, diameter, m<br />
e specific energy, J/kg · K<br />
h heat <strong>transfer</strong> coefficient, W/m 2 · K, specific enthalpy, J/kg<br />
k thermal conductivity, W/m · K<br />
L path length, m<br />
q heat flow, W<br />
q’’ heat flux, W/m 2<br />
R thermal resistance, K/W<br />
r radius, m<br />
S surface area, m 2<br />
T temperature, K<br />
t time, s<br />
V volume, m 3<br />
Vˆ velocity, m/s<br />
Greek Letter Symbols<br />
β coefficient of volumetric expansion, m -1<br />
∆ change, dimensionless<br />
δ thickness, m<br />
µ dynamic viscosity, N/m · s<br />
ν kinematic viscosity, m 2 /s<br />
ρ density, kg/m 3<br />
Roman Letter Subscripts<br />
cd conduction<br />
cv convection<br />
f fluid<br />
s surface condition<br />
w wall condition<br />
1
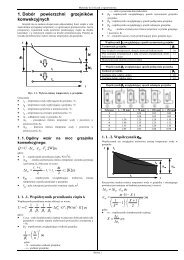
Introduction<br />
Thermal sciences generally deal with four basic thermal transport modes:<br />
conduction, convection, phase change, and radiation.<br />
The process by which heat diffuses through a solid or a stationary fluid is<br />
termed heat conduction. Situations in which heat <strong>transfer</strong> from a wetted surface is<br />
assisted by the motion of the fluid give rise to heat convection, and when the fluid<br />
undergoes a liquid–solid or liquid–vapor state transformation at or very near the<br />
wetted surface, attention is focused on this phase-change heat <strong>transfer</strong>. The<br />
exchange of heat between surfaces, or between a surface and a surrounding fluid, by<br />
long-wavelength electromagnetic radiation is termed thermal heat radiation.<br />
During this short course I describe briefly these modes of heat <strong>transfer</strong>, with<br />
emphasis on an important parameter known as the thermal resistance to heat<br />
<strong>transfer</strong>. The calculation methods presented here will be relatively simple to give you<br />
just basis for the further study at this faculty, as for example heat or ventilation<br />
installations design.<br />
The different types of heat <strong>transfer</strong><br />
In thermodynamics, heat is defined as the energy that crosses the boundary of<br />
a system when this energy transport occurs due to a temperature difference between<br />
the system and its surroundings. The second law of thermodynamics states that heat<br />
always flows over the boundary of the system in the direction of falling temperature.<br />
However, thermodynamics does not state how the heat <strong>transfer</strong>red depends on this<br />
temperature driving force, or how fast or intensive this irreversible process is. It is the<br />
task of the science of heat <strong>transfer</strong> to clarify the laws of this process.<br />
Three modes of heat <strong>transfer</strong> can be distinguished: conduction, convection,<br />
and radiation. The following course deal with their basic laws and phenomenological<br />
description of heat <strong>transfer</strong> processes, using the thermodynamic concepts of<br />
temperature, heat, heat flow and heat flux. In contrast to thermodynamics, which<br />
mainly deals with homogeneous systems, the so-called phases, heat <strong>transfer</strong> is a<br />
continuum theory which deals with fields extended in space and also dependent on<br />
time.<br />
This has consequences for the concept of heat, which in thermodynamics is<br />
defined as energy which crosses the system boundary. In heat <strong>transfer</strong> one speaks<br />
of a heat flow also within the body. This contradiction with thermodynamic<br />
terminology can be resolved by considering that in a continuum theory the mass and<br />
volume elements of the body are taken to be small systems, between which energy<br />
can be <strong>transfer</strong>red as heat. Therefore, when one speaks of heat flow within a solid<br />
body or fluid, or of the heat flux vector field in conjunction with the temperature field,<br />
the thermodynamic theory is not violated.<br />
As in thermodynamics, the thermodynamic temperature T is used in heat<br />
<strong>transfer</strong>. However with the exception of radiative heat <strong>transfer</strong> the zero point of the<br />
thermodynamic temperature scale is not needed, usually only temperature<br />
differences are important. For this reason a thermodynamic temperature with an<br />
adjusted zero point, an example being the Celsius temperature, is used.<br />
<strong>Heat</strong> conduction<br />
<strong>Heat</strong> conduction is the <strong>transfer</strong> of energy between neighbouring molecules in a<br />
substance due to a temperature gradient. In metals also the free electrons <strong>transfer</strong><br />
energy. In solids which do not transmit radiation, heat conduction is the only process<br />
2
for energy <strong>transfer</strong>. In gases and liquids heat conduction is superimposed by an<br />
energy transport due to convection and radiation.<br />
The mechanism of heat conduction in solids and fluids is difficult to understand<br />
theoretically. We do not need to look closely at this theory; it is principally used in the<br />
calculation of thermal conductivity, a material property. We will limit ourselves to the<br />
phenomenological discussion of heat conduction, using the thermodynamic<br />
quantities of temperature, heat flow and heat flux, which are sufficient to deal with<br />
most technically interesting conduction problems.<br />
One-Dimensional Conduction<br />
Thermal diffusion through solids is governed by Fourier’s law (Jean Baptiste<br />
Fourier (1768–1830) was Professor for Analysis at the Ecole Polytechnique in Paris<br />
and from 1807 a member of the French Academy of Science. His most important<br />
work “Th´eorie analytique de la chaleur” appeared in 1822. It is the first<br />
comprehensive mathematical theory of conduction and cointains the “Fourier Series”<br />
for solving boundary value problems in transient heat conduction.), the basic law for<br />
the conduction of heat, from 1822. The minus sign in this equation is accounting for<br />
the 2nd law of thermodynamics: heat flows in the direction of falling temperature.<br />
Fourier’s law in one-dimensional form is expressible as:<br />
where q is the heat current, k the thermal conductivity of the medium, A the<br />
crosssectional area for heat flow, and dT/dx the temperature gradient, which,<br />
because it is negative, requires insertion of the minus sign to assure a positive heat<br />
flow q. The temperature difference resulting from the steady-state diffusion of heat is<br />
thus related to the thermal conductivity of the material, the cross-sectional area A,<br />
and the path length L, according to<br />
The form of above equation, where k and A are presumed constant, suggests<br />
that in a way that is analogous to Ohm’s law governing electrical current flow through<br />
a resistance, it is possible to define a conduction thermal resistance as<br />
3
Convective <strong>Heat</strong> Transfer<br />
<strong>Heat</strong> Transfer Coefficient Convective thermal transport from a surface to a<br />
fluid in motion can be related to the heat <strong>transfer</strong> coefficient h, the surface-to-fluid<br />
temperature difference, and the “wetted” surface area S in the form<br />
The differences between convection to a rapidly moving fluid, a slowly flowing<br />
fluid, or a stagnant fluid, as well as variations in the convective heat <strong>transfer</strong> rate<br />
among various fluids, are reflected in the values of h. For a particular geometry and<br />
flow regime, h may be found from available empirical correlations and/or theoretical<br />
relations. Use of above equation makes it possible to define the convective thermal<br />
resistance as<br />
4
Natural Convection<br />
In natural convection, fluid motion is induced by density differences resulting<br />
from temperature gradients in the fluid. The heat <strong>transfer</strong> coefficient for this regime<br />
can be related to the buoyancy and the thermal properties of the fluid.<br />
Forced Convection<br />
Here fluid motion in tubes, pipes or channels with the equivalent diameter de is<br />
forced by an external machine such as fun or pump.<br />
Coordinate systems<br />
<strong>Heat</strong> <strong>transfer</strong> and fluid flow analyses of objects of various sizes and shapes<br />
and their corresponding flow fields are facilitated by working in a coordinate system<br />
that provides a good fit to the flow geometry.<br />
Most practically important application, is the conduction of heat independent of<br />
time, so called steady conduction, in a flat plate or in a hollow cylinder. The<br />
assumption is made that heat flows in only one direction, perpendicular to the plate<br />
surface, and radially in the cylinder and sphere. The temperature field is then only<br />
dependent on one geometrical coordinate. This is known as one-dimensional heat<br />
conduction.<br />
5