Topics in HIV Medicine® - International AIDS Society-USA
Topics in HIV Medicine® - International AIDS Society-USA
Topics in HIV Medicine® - International AIDS Society-USA
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>International</strong> <strong>AIDS</strong> <strong>Society</strong>–<strong>USA</strong><br />
<strong>Topics</strong> <strong>in</strong> <strong>HIV</strong> Medic<strong>in</strong>e<br />
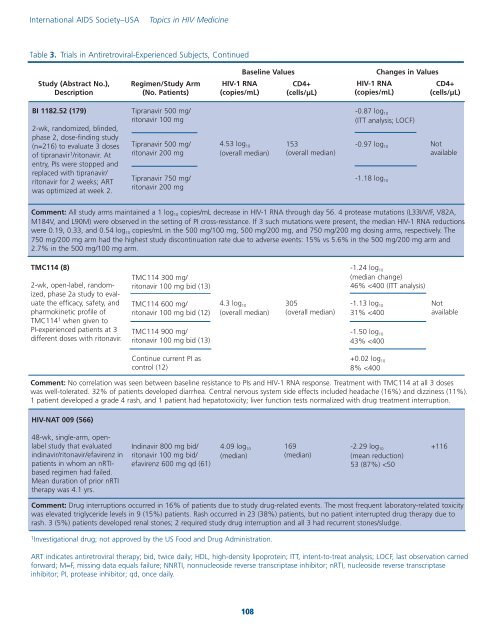
Table 3. Trials <strong>in</strong> Antiretroviral-Experienced Subjects, Cont<strong>in</strong>ued<br />
Study (Abstract No.),<br />
Description<br />
Regimen/Study Arm<br />
(No. Patients)<br />
<strong>HIV</strong>-1 RNA<br />
(copies/mL)<br />
Basel<strong>in</strong>e Values<br />
CD4+<br />
(cells/µL)<br />
<strong>HIV</strong>-1 RNA<br />
(copies/mL)<br />
Changes <strong>in</strong> Values<br />
CD4+<br />
(cells/µL)<br />
BI 1182.52 (179)<br />
2-wk, randomized, bl<strong>in</strong>ded,<br />
phase 2, dose-f<strong>in</strong>d<strong>in</strong>g study<br />
(n=216) to evaluate 3 doses<br />
of tipranavir 1 /ritonavir. At<br />
entry, PIs were stopped and<br />
replaced with tipranavir/<br />
ritonavir for 2 weeks; ART<br />
was optimized at week 2.<br />
Tipranavir 500 mg/<br />
ritonavir 100 mg<br />
Tipranavir 500 mg/<br />
ritonavir 200 mg<br />
Tipranavir 750 mg/<br />
ritonavir 200 mg<br />
4.53 log 10<br />
(overall median)<br />
153<br />
(overall median)<br />
-0.87 log 10<br />
(ITT analysis; LOCF)<br />
-0.97 log 10<br />
-1.18 log 10<br />
Not<br />
available<br />
Comment: All study arms ma<strong>in</strong>ta<strong>in</strong>ed a 1 log 10 copies/mL decrease <strong>in</strong> <strong>HIV</strong>-1 RNA through day 56. 4 protease mutations (L33I/V/F, V82A,<br />
M184V, and L90M) were observed <strong>in</strong> the sett<strong>in</strong>g of PI cross-resistance. If 3 such mutations were present, the median <strong>HIV</strong>-1 RNA reductions<br />
were 0.19, 0.33, and 0.54 log 10 copies/mL <strong>in</strong> the 500 mg/100 mg, 500 mg/200 mg, and 750 mg/200 mg dos<strong>in</strong>g arms, respectively. The<br />
750 mg/200 mg arm had the highest study discont<strong>in</strong>uation rate due to adverse events: 15% vs 5.6% <strong>in</strong> the 500 mg/200 mg arm and<br />
2.7% <strong>in</strong> the 500 mg/100 mg arm.<br />
TMC114 (8)<br />
2-wk, open-label, randomized,<br />
phase 2a study to evaluate<br />
the efficacy, safety, and<br />
pharmok<strong>in</strong>etic profile of<br />
TMC114 1 when given to<br />
PI-experienced patients at 3<br />
different doses with ritonavir.<br />
TMC114 300 mg/<br />
ritonavir 100 mg bid (13)<br />
TMC114 600 mg/<br />
ritonavir 100 mg bid (12)<br />
TMC114 900 mg/<br />
ritonavir 100 mg bid (13)<br />
4.3 log 10<br />
(overall median)<br />
305<br />
(overall median)<br />
-1.24 log 10<br />
(median change)<br />
46%