Discovering critical residues in glutathione reductase - Gentoo
Discovering critical residues in glutathione reductase - Gentoo
Discovering critical residues in glutathione reductase - Gentoo
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
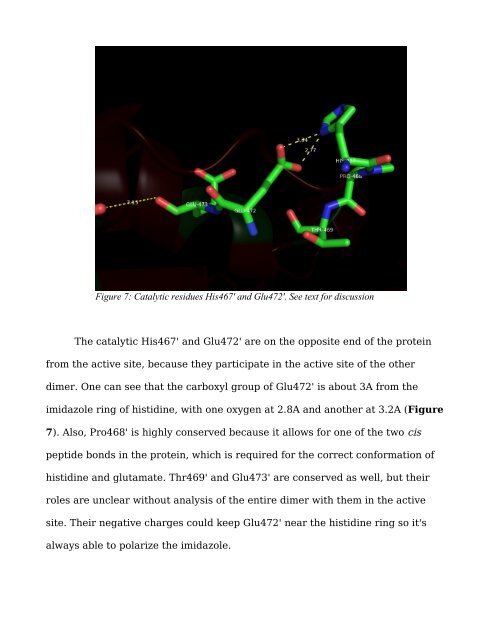
Figure 7: Catalytic <strong>residues</strong> His467' and Glu472'. See text for discussion<br />
The catalytic His467' and Glu472' are on the opposite end of the prote<strong>in</strong><br />
from the active site, because they participate <strong>in</strong> the active site of the other<br />
dimer. One can see that the carboxyl group of Glu472' is about 3A from the<br />
imidazole r<strong>in</strong>g of histid<strong>in</strong>e, with one oxygen at 2.8A and another at 3.2A (Figure<br />
7). Also, Pro468' is highly conserved because it allows for one of the two cis<br />
peptide bonds <strong>in</strong> the prote<strong>in</strong>, which is required for the correct conformation of<br />
histid<strong>in</strong>e and glutamate. Thr469' and Glu473' are conserved as well, but their<br />
roles are unclear without analysis of the entire dimer with them <strong>in</strong> the active<br />
site. Their negative charges could keep Glu472' near the histid<strong>in</strong>e r<strong>in</strong>g so it's<br />
always able to polarize the imidazole.