Volume 12 - IVFOnline.com
Volume 12 - IVFOnline.com
Volume 12 - IVFOnline.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ESHRE 2010, Rome, Italy<br />
VOLUME <strong>12</strong>
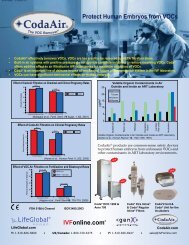
FDA 510(k) Cleared
CONTENTS<br />
Fertility Magazine<br />
The First Magazine In Fertility TM<br />
FEATURED ON THE COVER<br />
Belgian legislation regarding IVF<br />
by Guy De Groote, MD, Frank Vandekerckhove, MD . . . . . . . . . . . . .9<br />
54<br />
Successful vitrification in a closed carrier device of blastocysts<br />
originating from infertile patients, egg donors, or in-vitro<br />
maturation by J Lopez, NH Zech, P Frias, P Vanderzwalmen . . . . .40<br />
Automated Robotic Human ICSI<br />
by Navid Esfandiari, DVM, PhD, HCLD, Zhe Lu, PhD, Xuping<br />
Zhang, PhD, Robert F. Casper, MD, Yu Sun, PhD . . . . . . . . . . . . . . .46<br />
Improving air quality in ART laboratories by Giles Palmer . . . . . .54<br />
Too Much of a Good Thing by Courtney Sirotin . . . . . . . . . . . . . . . .78<br />
Improving air quality<br />
in ART laboratories<br />
FEATURES<br />
<strong>12</strong> Efficiency of human oocyte slow freezing: results from five assisted reproduction centres<br />
by L Parmegiani, F Bertocci, C Garello, MC Salvarani, G Tambuscio, R Fabbri<br />
22 Effect of growth hormone on oocyte <strong>com</strong>petence in patients with multiple IVF failures<br />
by A Hazout, AM Junca, Y Ménézo, J de Mouzon, P Cohen-Bacrie<br />
32 S3 Vitrification System: a Novel Approach to Blastocyst Freezing by<br />
James J. Stachecki, PhD, Jacques Cohen, PhD<br />
44 Life-long impact of the first cell cycle by Dmitri Dozortsev, MD, PhD<br />
49 An Alternate Method For Shipping Sperm: Aerogel Containers Fitted<br />
With Data Loggers by Lakshmi Sharma, MSc, MPhil, ELD,<br />
Susan Tarchala, MS, TS, Jon Nakagawa, CPA, John Perry, BS, MBA,<br />
Richard Rawlins, PhD, HCLD<br />
44<br />
Life-long impact<br />
of the first cell<br />
4 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
52 Epigenetics and the ART Lab by Sangita Jindal, PhD, HCLD<br />
58 The use of birefringence technology in assisted human reproduction<br />
by Simon J Phillips, MSc, BSc<br />
64 The identification of a toxic substance in the in vitro fertilization laboratory: the value of interlaboratory<br />
<strong>com</strong>munication by Tom Turner, MS ELD (ABB)<br />
66 Clinical Implementation of the Halosperm ® Test Kit in Combination with SCA ®<br />
by Kellie Williams, PhD and J. Kevin Thibodeaux, PhD, HCLD<br />
69 A Prospective Randomized Comparison of global ® Medium with Sequential Media for<br />
Culture of Human Embryos to the Blastocyst Stage by Don Rieger, PhD<br />
72 “Morning Sickness” – An overview by Michele Brown, MD, FACOG<br />
75 Vitamin D Deficiency and Pregnancy by Michele Brown, MD, FACOG<br />
84 New Products<br />
86 Books<br />
87 Resources<br />
92 Conferences<br />
Instructions to<br />
Contributors<br />
To submit an Article/Abstract<br />
email us at: editor@IVFonline.<strong>com</strong><br />
Note: Articles and Abstracts must<br />
be ac<strong>com</strong>panied<br />
by a photo of the author(s).<br />
To submit an Ad email us at:<br />
advertise@IVFonline.<strong>com</strong><br />
Editor in Chief: Monica Mezezi, MBA<br />
Editor(s): Don Rieger, PhD<br />
Assistant Editor: Michael West<br />
Design: Debrah Frank<br />
Editorial Office: IVFonline, 24 Norwich St. E.<br />
Guelph, Ontario, Canada, N1H 2G6<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
Email: editor@IVFonline.<strong>com</strong><br />
www.IVFonline.<strong>com</strong><br />
www.FertMag.<strong>com</strong><br />
Fertility Magazine and all its associates ©2010, All Rights Reserved. Covers, contents, images, ads in print or web form are copryight protected and reprinting or reproduction of any<br />
kind is expressly prohibited without the written permission of Fertility Magazine. Fertility Magazine does not knowingly accept false or misleading advertising, articles, opinions or<br />
editorial, nor does the publisher assume any responsibility for the consequences that occur should any such material appear, and assumes no responsibility for content, text, opinions<br />
or artwork of advertisements appearing in Fertility Magazine in print or web form. Some of the views expressed by contributors may not be the representative views of the publisher.<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
5
StemQ® Hepatic Model System<br />
Your Hepatocyte Solution<br />
Zenith Biotech’s StemQ® Hepatic Model System includes hormonally defined<br />
media, HepatoGro for growth of hepatic stem cells, HepatoDiff for<br />
expansion of hepatoblasts and differentiation into hepatocytes and<br />
HepatoMain for maintenance of mature hepatocytes. Stem cells <strong>com</strong>mitted to<br />
the endodermal and hepatic lineage plated on MatriPlus Liver Biomatrix<br />
rapidly differentiate into hepatocytes. Primary and stem cell-derived<br />
hepatocytes maintain metabolic functions longer when plated on MatriPlus<br />
Liver Biomatrix <strong>com</strong>pared to Collagen I, maintained in HepatoMain<br />
Maintenance Medium.<br />
Human Hepatocytes<br />
Primary Hepatocytes<br />
Stem Cell-Derived Hepatocytes<br />
Human hepatocytes on Liver Biomatrix in<br />
HepatoMain.<br />
StemQ® Hepatic Model System<br />
• HepatoGro Growth Media<br />
• HepatoDiff Differentiation Media<br />
• HepatoMain Maintenance Media<br />
• MatriPlus Liver Biomatrix<br />
– Isolated from decellularized liver tissue<br />
– Liver matrix biochemistry retained<br />
– Matrix scaffold reduced to μm sized<br />
particles in suspension<br />
– Matrix Particle suspension coated onto<br />
multi-well plates<br />
• Fresh and cryopreserved hepatocytes attach to<br />
Liver Biomatrix within 10 minutes<br />
• Fresh and cryopreserved hepatocytes plated<br />
onto Liver Biomatrix sustain function and<br />
morphology longer than on Collagen I<br />
www.zenithbiotech.<strong>com</strong>
zenith biotech<br />
StemQ® ES Cell Products<br />
Zenith<br />
Stem Cells<br />
Biotech<br />
& Reagents<br />
StemQ<br />
for Research & Drug Discovery<br />
® branded products consist of cells, media and reagents which are application tested.<br />
Produced in an FDA registered, ISO certified facility, we do the qualification so you do not have to. ES Cells<br />
have proven germ line transmission and differentiation potential.<br />
Embryonic Stem Cells<br />
• Proven Germline Transmission<br />
• 40xy Karyotype<br />
• Sterility, Mycoplasma & Viral Tested<br />
DBA Chimera<br />
Primary Murine Embryonic Fibroblast<br />
(PMEFs) Feeders<br />
• Derived from <strong>12</strong>.5 dpc embryos<br />
• Passage 3 (2.5-3 doublings between passages)<br />
• Sterility, Mycoplasma & Viral Tested<br />
• Untreated or growth arrested<br />
ES Qualified Media and Reagents<br />
• Formulations optimized for growth and maintenance of<br />
ES cell pluripotency (Mouse and Human ES Cells)<br />
• Each lot tested and qualified on ES Cells<br />
StemQ ® Murine Embryonic Stem Cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Catalogue #<br />
Strain <strong>12</strong>9 svev , 2 vials 2.5 X 10 6 cells per vial . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ES<strong>12</strong>9<br />
Strain C57/BL6, 2 vials 2.5 X 10 6 cells per vial . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ESC57<br />
Strain DBA/1, 2 vials 2.5 X 10 6 cells per vial . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ESDBA<br />
Proven germ line transmission. All cells are sterility, Mycoplasma and virus tested. Cell lines are karyotyped<br />
> 85% normal. Provided as 2 vials containing 2.5 X 10 6 cells per vial.<br />
StemQ ® Primary Murine Embryonic Fibroblasts (PMEFs) Feeder Cells . . . . . . .Catalogue #<br />
Zenith CF-1 PMEFs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ZFVC-001<br />
Zenith CF-1 PMEFs, growth arrested . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ZFGA-002<br />
Zenith Neomycin resistant PMEFs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .NRVC-003<br />
Zenith Neomycin resistant PMEFs, growth arrested . . . . . . . . . . . . . . . . . . . . . . . . . . NRGA-004<br />
Cells are derived from <strong>12</strong>.5-13 day embryos to be used as feeder layer for Murine and Human ES cells. Provided<br />
as 5 vials 5 X 10 6 cells per vial. All cells are passage 3 (approx. 2-2.5 doublings per passage), and<br />
are virus, mycoplasma and sterility tested.<br />
www.zenithbiotech.<strong>com</strong>
FREE SUBSCRIPTION<br />
Free Subscription…<br />
Subscribe today to Fertility Magazine …”The First Magazine In Fertility TM ”<br />
By joining us today you will receive the latest in:<br />
• International News • Scientific Information• Patient Corner’s • New Products<br />
All the information needed to keep you up-to-date in Fertility.<br />
Join Now …..Join Today<br />
Call US/Canada: 1-800-720-6375, International: 1-519-826-5800, email: subscribe@IVFonline.<strong>com</strong>,<br />
or Fax the form below to: 1-519-826-6947.<br />
Yes …Sign me up!<br />
To receive Fertility Magazine …”The First Magazine In Fertility TM ”<br />
Name:<br />
Address:<br />
City: State: Zip Code: Country:<br />
Email address:<br />
Send it to a Friend at email:
INTERNATIONAL NEWS<br />
Belgian legislation regarding IVF<br />
by Guy De Groote, MD, Lab Director, CRI Laboratories, Gent, Belgium<br />
Frank Vandekerckhove, MD, gynaecologist, Fertility Center, Ghent University, Gent, Belgium<br />
In-vitro fertilization (IVF) units were first established in<br />
Belgium in the 1980’s as private initiatives of interested<br />
clinicians and laboratory directors, both in hospitals<br />
and in private institutions. We all had the intention to help<br />
couples with fertility problems using this exciting new<br />
procedure. As the number of treatments grew and the<br />
visibility of the new technique increased, IVF units also<br />
became more professional, incorporating quality<br />
standards. Almost as a side effect, they became subject to<br />
new legislation.<br />
The original legislation (1999) was not concerned with<br />
medical practice or quality. Rather, it was intended to limit<br />
the number of centers and the health care expenditure<br />
related to the new treatment. The first quality standards<br />
were suggested by the profession itself (e.g. the Flemish<br />
Society of Clinical Embryologists, VVKE), anticipating<br />
legislation.<br />
As the possible applications of IVF related techniques<br />
became clear, a public ethical and philosophical debate<br />
arose. Health care workers in the field, together with local<br />
ethical <strong>com</strong>mittees, had to cope with many difficult issues,<br />
including age limits for patients and donors, a growing<br />
number of spare embryos, the use of embryos for stem cell<br />
research, sex selection, cloning, and remuneration of<br />
donors. Of course, the decisions were often influenced by<br />
pressure from the patient couple. The legislation on<br />
ethical issues (2003 and 2007) provided answers to these<br />
important questions, adding some strict rules, and partly<br />
limiting the possibilities for health care and research.<br />
Subsequent legislation also included quality<br />
requirements. An important issue was single embryo<br />
transfer (2003) in order to limit the health problems and<br />
expenditure created by the increasing number of twins<br />
and triplets.<br />
The law of 2008 put IVF and intrauterine insemination<br />
together with stem cell applications in a general legal<br />
framework for human tissue banking. This law<br />
introduced stringent quality requirements, established<br />
control procedures by the national drug administration,<br />
and incorporated the existing European directives.<br />
In the following sections, I will try to summarize the<br />
most important laws and royal decrees (RD) involved. For<br />
full details the interested reader is referred to the original<br />
publications in the Official Journal available through<br />
http://www.just.fgov.be.<br />
Guy De Groote, MD<br />
Frank Vandekerckhove, MD<br />
1. Creation of centers for reproductive medicine (RDs<br />
Feb. 15, 1999)<br />
• Incorporation of IVF units in centers for<br />
reproductive medicine<br />
• Limitation of the number of IVF centers<br />
• Exclusion of extramural IVF centers<br />
• Mandatory registration<br />
This first legislation limited the (growing) number of<br />
IVF centers and thus the health expenditure related to it.<br />
This legislation incorporated the existing IVF units into<br />
a limited number of newly-created hospital-based centers<br />
for reproductive medicine. So-called type-B centers for<br />
reproductive medicine were allowed to perform all IVF<br />
related activities, whereas the activity in type-A centers was<br />
mostly limited to procedures up to the stage of oocyte<br />
pickup. For embryo culture, freezing, and transfer, type-A<br />
centers must transfer the ova to a type-B center. Therefore,<br />
every type-A center must have a formal agreement with a<br />
type-B center.<br />
The number of both types of IVF centers was limited;<br />
two type-B IVF centers are allowed per province (1<br />
university-based, 1 other), and one type-A center was<br />
allowed per 700,000 inhabitants. Belgium consists of 10<br />
provinces and has a population of approximately 10 million<br />
(2009) inhabitants. Today, there are 18 type-B, and 15 type-A,<br />
registered IVF centers. Their activity in the last published<br />
CONTINUED ON PAGE 10<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
9
INTERNATIONAL NEWS<br />
CONTINUED FROM PAGE 9<br />
report (data from 2007) totaled 26,836 cycles, of which 18,025<br />
(67%) involved fresh transfers and 7,197 (27%) involved<br />
transfer of frozen embryos.<br />
This RD introduced obligatory multidisciplinary<br />
cooperation, including psychological and social assistance,<br />
surgery, andrology, and echography. The requirements for<br />
Type-B type centers also included microsurgery and<br />
reproductive endocrinology, and cooperation with a center<br />
for medical genetics. Registration of data and participation in<br />
a quality assessment program became obligatory. To this<br />
end, participation in the Belgian Register for Assisted<br />
Procreation (www.belrap.be) became mandatory for IVF<br />
centers, ten years after this register had been started (1989).<br />
2. Law on embryo research in vitro (Law May 11, 2003)<br />
• Research requires agreement with university<br />
based center for reproductive medicine and<br />
specific approvals<br />
• Embryo research allowed up to day 14 of<br />
development<br />
• Strictly forbids: sex selection, eugenics,<br />
reproductive cloning, <strong>com</strong>mercial use of embryos<br />
According to this law embryo research is allowed on<br />
embryos up to day 14 of development (disregarding a<br />
possible freezing period) if needed for therapeutic<br />
purposes or research contributing to better knowledge in<br />
human (in)fertility, transplantation or diseases. This<br />
research must be linked with one of the university-based<br />
centers for reproductive medicine or medical genetic<br />
departments, is subject to approval by an ethical<br />
<strong>com</strong>mittee, and is controlled by a federal <strong>com</strong>mission for<br />
embryo research.<br />
The defined penalties are severe, including large<br />
monetary penalties and/or detention up to 5 years and/or<br />
the prohibition from performing any medical activity or<br />
research for 5 years.<br />
This law clearly forbids reproductive human cloning,<br />
implantation of human embryos into animals (some<br />
limited exceptions), any <strong>com</strong>mercial use or application for<br />
eugenics or sex selection (exception for sex linked<br />
diseases).<br />
3. Reimbursement and single embryo transfer (RD<br />
June 4, 2003)<br />
• Reimbursement for IVF laboratory work<br />
(maximum of. 6 cycles, limit of. 42 years of age)<br />
• Single embryo transfer mandatory in first and (if<br />
good quality) second cycles<br />
This royal decree introduced reimbursement by the<br />
national health insurance system (covering more than 95<br />
% of the population) of laboratory procedures for IVF,<br />
under specific conditions of patient age and the number of<br />
embryos transferred.<br />
For women up to 35 years of age: single embryo<br />
transfer mandatory in the first treatment cycle. In the<br />
second cycle one or two ( depending on quality) embryos<br />
can be replaced. In the third and subsequent fresh cycles,<br />
or in any frozen embryo transfer cycles, a maximum 2<br />
embryos may be replaced. For older women up to 39 years<br />
of age, a maximum 2 embryos can be transferred in the<br />
first or second cycle and 3 in following cycles. For women<br />
older than 39 years of age, no maximum number is<br />
applied. The reimbursement is only applied for women<br />
up to 42 years of age, and for a maximum of 6 IVF<br />
treatment cycles.<br />
This reimbursement (1182 EUR) covers all laboratory<br />
expenses for regular IVF procedures (including germ cell<br />
selection, insemination, embryo culture and evaluation,<br />
and cryopreservation) and must be applied for all treated<br />
patients covered by the Belgian national health insurance<br />
system.<br />
Later RD’s introduced a reimbursement for<br />
gonadotrophin treatment (RD's of Sept 14 and 15, 2006,<br />
RD of Oct 6, 2008, RD Dec 17, 2008), limited to 6 IVF cycles<br />
and 6 IUI or other cycles each for (clomiphene resistant)<br />
patients less than 43 years of age. A RD of July 2, 2008<br />
included reimbursement for intrauterine or intracervical<br />
insemination, including an obligatory registration.<br />
4. Fate of supernumerary embryos and gametes (Law<br />
of Jul 6, 2007)<br />
• IVF, gamete and embryo freezing limited to<br />
centers for reproductive medicine<br />
• Maximum age limit to 45 years (pick-up) and to<br />
47 years of age (transfer)<br />
• Decision on the fate of supernumerary embryo’s:<br />
personal use, donation or research<br />
• Donation of gametes or embryos allowed, limited<br />
to 6 successful recipients<br />
• Preimplantation diagnostics on embryos allowed<br />
• Strictly forbidden: eugenics or sex selection<br />
(except for sex linked diseases)<br />
• Embryo matching allowed<br />
This law set a clear age limit of 45 years for oocyte<br />
recovery and 47 years of age for embryo transfer. If a<br />
woman has her own good-quality frozen embryo’s<br />
10 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
INTERNATIONAL NEWS<br />
available, then these must be used first before proceeding<br />
to a next fresh cycle. Oocyte recovery from under-age girls<br />
is permitted if required for medical reasons.<br />
This law includes details on the information to be<br />
provided to the patient, psychological support, and the<br />
contract to be signed before proceeding. The last should<br />
include the patient’s decision on the destination of<br />
supernumerary embryos: personal use, destruction,<br />
research, or donation. The contract also stipulates the fate<br />
of frozen embryos in case of death or divorce. If the couple<br />
decides for personal use by the surviving partner, then<br />
these embryos can only be used for this purpose in the<br />
period form 6 months to 2 years after the death of the first<br />
partner. Gametes (oocytes, sperm or gonadal fragments)<br />
may be collected for personal use, donation or research. A<br />
similar contract is required.<br />
The normal storage period for frozen embryos for<br />
personal use is 5 years, for gametes 10 years (extension<br />
possible). Embryo donation for eugenic purposes or sex<br />
selection is not allowed. Embryos or gametes from one<br />
donor may be used to produce a pregnancy in a maximum<br />
of 6 recipients. Anonymity is mandatory and this law<br />
absolutely excludes right of inheritance between donor<br />
and receiving family or child. The <strong>com</strong>mercial use of<br />
gametes or embryos is not allowed.<br />
Preimplantation diagnostics is allowed except for<br />
eugenic purposes or sex selection (except in case of sex<br />
linked hereditary diseases). The number of centers for<br />
preimplantation diagnostics can be limited, but should not<br />
be less than 8. Matching of embryos is allowed.<br />
As for the law on embryo research severe penalties are<br />
applicable. An official organization for control and<br />
inspections is established (Law of Jul 24, 2008, RD Dec. 17,<br />
2008 and Sept 20, 2009).<br />
5. IVF and stem cell research included in a general law<br />
on tissue banking for human application or<br />
scientific research. (Law Dec 19, 2008 and Dec 23,<br />
2009, several RD's Sep 28, 2009, MD Oct 14, 2009)<br />
• Creation of tissue banks and related structures,<br />
mostly limited to hospital environment<br />
• Application: all aspects (extraction, storage,<br />
processing, distribution and use) of stem cell<br />
banking, reproductive applications (including<br />
IVF, intra-uterine insemination, donations), any<br />
human tissue materials for human application or<br />
scientific research<br />
• Detailed quality requirements, detailed<br />
registration requirements, <strong>com</strong>munication of<br />
adverse events and non conformities to the<br />
authorities<br />
This extensive law and related decrees introduced the<br />
structures for tissue banking in detail: obtaining,<br />
extraction, processing, storage, distribution and use of any<br />
human material or tissues for human therapeutic<br />
application or scientific research. The application is very<br />
large, including any kind of tissue and cells (i.e. any<br />
collection of human cells that is not linked by fibrous<br />
tissue), IVF, and every application of stem cells. Excluded<br />
are organ transplantation and blood transfusion (both<br />
subject of a separate legislation), immediate autologous<br />
treatment (without processing) and direct diagnostic use<br />
of the material for the person involved. Some tissues (hair,<br />
nails, urine, feces, sweat, tears, and mother milk) are<br />
excluded from this law.<br />
This law established three different categories of<br />
institutions: tissue banks for human body materials,<br />
intermediary structures, and production units. The centers<br />
for reproductive medicine are considered to be tissue<br />
banks and remain the only structures allowed to deal with<br />
gametes or embryos. Tissue banks must always be situated<br />
in a hospital.<br />
Part of the activity can be done in intermediary<br />
structures (processing, storage, distribution), or in a<br />
production unit. These should have a contractual link with<br />
a tissue bank. If they are recognized as an intermediary<br />
structure, then medical laboratories can perform<br />
capacitation of sperm for partner donation.<br />
Tissue banking without a preventive, therapeutic or<br />
diagnostic purpose, or lacking a relevant scientific target<br />
(as confirmed by the opinion of an ethics <strong>com</strong>mittee) is not<br />
allowed.<br />
Several royal decrees provided several additions to the<br />
law: detailing quality requirements, infrastructure, cleanroom<br />
specifications (including EU Grade A working<br />
environment and Grade D background environment),<br />
quality assurance, donor selection criteria, safety,<br />
exclusion of risk of virus transmission, full traceability,<br />
registration (data should be kept for 30 years), mandatory<br />
<strong>com</strong>munication of serious adverse effects, and nonconformities.<br />
Approval of a tissue bank is for maximum 4 years with<br />
a extensive inspection at least every 2 years. Control and<br />
inspection organizations (mostly the federal drug<br />
administration FAGG/AFMPS) are nominated.<br />
You can contact Guy De Groote at: gdg@cri.be<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
11
INTERNATIONAL NEWS<br />
Efficiency of human oocyte slow freezing: results<br />
from five assisted reproduction centres<br />
L Parmegiani 1,7 , F Bertocci 2 , C Garello 3 , MC Salvarani 4 , G Tambuscio 5 , R Fabbri 6<br />
1 Reproductive Medicine Unit, GynePro Medical Centres, Bologna, Italy; 2 Chianciano Salute, Centre for Reproductive Health,<br />
Chianciano Terme (SI), Italy; 3 Livet Clinic, Turin, Italy; 4 Centre for Reproductive Medicine, Department of Obstetrics, Gynecology<br />
and Neonatology, University of Parma, Parma, Italy; 5 Department of Gynecological Science and Reproductive Medicine, University<br />
of Padua School of Medicine, Padua, Italy; 6 Human Reproductive Medicine Unit, University of Bologna, Bologna, Italy<br />
7 Correspondence: e-mail: l.parmegiani@gynepro.it<br />
c 2010, Reproductive Healthcare Ltd. Published by Elsevier Ltd. All rights reserved.<br />
Dr. Lodovico Parmegiani, 2009 Efficiency of human oocyte slow freezing: results from five assisted reproduction centres. Reprod<br />
BioMed Online, 18, 3, 352-359.<br />
Lodovico Parmegiani obtained his degree in Biology in 1996 from the University of<br />
Bologna and his specialization in Biochemistry and Clinical Chemistry in 2000 from<br />
the University of Modena and Reggio Emilia. He trained as a clinical embryologist<br />
at the Reproductive Endocrinology Centre, St Orsola Hospital, Bologna and<br />
received a research scholarship (2001–2007) from the University of Bologna. Since<br />
2002 he has been Laboratory Director, Reproductive Medicine Unit–GynePro<br />
Medical Centres, Bologna. In 2008 he received certification as Senior Clinical<br />
Embryologist from the European Society for Human Reproduction & Embryology.<br />
His current research interests are cryobiology, gamete selection and<br />
micromanipulation.<br />
DR LODOVICO PARMEGIANI<br />
Abstract<br />
It has been demonstrated previously that freezing oocytes within 2 h of retrieval increases the efficiency of cryopreservation via<br />
a slow-freezing/rapid-thawing protocol with 0.3 mol/l sucrose (SF/RT 0.3). The aim of this multicentre survey was to verify this<br />
observation on a larger scale. This was a retrospective study on the clinical out<strong>com</strong>e of 510 SF/RT 0.3 cycles divided into two<br />
groups: group A, freezing oocytes within 2 h of retrieval; group B, freezing oocytes more than 2 h after retrieval. The rate of bestquality<br />
embryos was significantly higher (33.24%) in group A than in group B (16.20%, P < 0.001). Pregnancy and implantation<br />
rates were 30.07% and 15.08% in group A versus 8.97% and 4.57% in group B (P < 0.001). Clinical pregnancy rates per thawed<br />
and per injected oocyte in group A were 5.53% and 10.41%, versus 1.46% and 2.77% in group B (P < 0.001). The overall yield<br />
from oocytes cryopreserved within 2 h of retrieval (group A) was 6.49 implantations per 100 oocytes thawed versus 1.74 for group<br />
B (P < 0.001). Embryo quality, pregnancy and implantation rates, and clinical efficiency of thawing cycles were all significantly<br />
improved when cryopreservation was carried out within 2 h of oocyte retrieval.<br />
Keywords: human, oocyte cryopreservation, slow freezing, timing of ICSI, 0.3 mol/l sucrose concentration<br />
Introduction<br />
Oocyte cryopreservation can be used in conjunction<br />
with conventional IVF, representing, as it does, an<br />
alternative which circumvents many of the ethical issues<br />
associated with embryo cryopreservation. Oocyte freezing<br />
also allows extension or preservation of fertility in women<br />
who intend to delay motherhood for family planning<br />
reasons or in those at risk of losing their gonadal function.<br />
While in Italy the adoption of oocyte cryopreservation as<br />
a clinical tool represents the only alternative to embryo<br />
cryopreservation, which is forbidden by the national IVF<br />
<strong>12</strong> FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
INTERNATIONAL NEWS<br />
law (Benagiano and Gianaroli, 2004), in other situations,<br />
‘egg cryo-banking’ could represent a more efficient<br />
approach in egg donor–recipient treatment. The early<br />
successes using human cryopreserved oocytes were<br />
reported 20 years ago (Chen, 1986; Al-Hasani et al., 1987)<br />
but for a long period this technology was not investigated<br />
further, being perceived as inefficient and unsafe. The<br />
publication of later studies optimizing various<br />
cryopreservation techniques (Gook et al., 1993, 1994,<br />
1995a,b; Porcu et al., 1997; Kuleshova et al., 1999; Fabbri et<br />
al., 2001; Kuwayama et al., 2005) opened new perspectives<br />
on oocyte cryopreservation. Nowadays, oocyte<br />
cryopreservation is seeing increasing clinical application<br />
worldwide. Some authors maintain that oocyte<br />
cryopreservation needs further studies on safety and<br />
efficiency. Furthermore, the Practice Committee of<br />
American Society for Reproductive Medicine (2006) has<br />
stated that this technique should be considered as<br />
experimental and that it should not be offered as a means<br />
to defer reproductive ageing.<br />
Most studies on human oocyte cryopreservation have<br />
used the slow-cooling–<strong>com</strong>puter-controlled protocol<br />
(slow-freezing/rapid-thawing), which is probably the<br />
main oocyte freezing method adopted in the majority of<br />
assisted reproduction centres. The results of oocyte<br />
cryopreservation using the slow freezing/rapid thawing<br />
(SF/RT) protocol with 1,2-propanediol (1,2-PROH) and<br />
high sucrose concentration (0.2 or 0.3 mol/l) as<br />
cryoprotectants have shown a gradual improvement in<br />
efficiency over time, with live birth rates per transfer<br />
increasing during recent years (Jain and Paulson, 2006).<br />
Furthermore, it has been demonstrated that the slowcooling<br />
technique with high sucrose concentration allows<br />
safe long-term oocyte cryopreservation (Yang et al., 2007;<br />
Parmegiani et al., 2008b).<br />
Oocyte freezing can be carried out up to several hours<br />
after retrieval; following thawing, the oocytes are cultured<br />
for a few hours before insemination to better evaluate the<br />
survival after the freezing/thawing procedure (Gook et al.,<br />
1994) and to allow the temperature-sensitive meiotic<br />
spindle to fully restore (Rienzi et al., 2004; Bianchi et al.,<br />
2005). The timing of intracytoplasmic sperm injection<br />
(ICSI) is a critical factor determining embryo viability and<br />
implantation as the developmental capacity of the oocyte<br />
declines some hours after oocyte retrieval (Yanagida et al.,<br />
1998). The optimal timing for insemination of fresh<br />
oocytes seems to be 37–41 h after human chorionic<br />
gonadotrophin (HCG) administration to trigger ovulation<br />
(Dozortsev et al., 2004). Similarly, metabolic ageing at ICSI<br />
of slow-cooled oocytes depends on the time of retrieval<br />
after HCG administration and on pre-incubation, but also<br />
on the post-thawing culture before insemination.<br />
Furthermore, it seems possible that the freezing procedure<br />
could influence oocyte ageing (Parmegiani et al., 2008a).<br />
In a previous study, it was demonstrated that freezing<br />
within 2 h from oocyte retrieval increases the efficiency of<br />
oocyte cryopreservation when using a slow freezing/rapid<br />
thawing protocol with 0.3 mol/l sucrose (SF/RT 0.3)<br />
(Parmegiani et al., 2008a). The aim of the present multicentre<br />
survey was to verify this result on a larger number<br />
of oocyte thawing cycles and to definitively establish the<br />
ideal time after oocyte retrieval for slow-cooling<br />
cryopreservation.<br />
Materials and methods<br />
Study population<br />
Five assisted reproduction centres in Italy were<br />
involved in this retrospective survey: three private centres<br />
(GynePro Medical Centres, Bologna; Livet Clinic, Turin;<br />
and Chianciano Salute, Chianciano Terme) and two<br />
University-based centres (Department of Gynecological<br />
Science and Reproductive Medicine, University of Padua;<br />
and Centre for Reproductive Medicine, University of<br />
Parma). In Italy, the insemination of more than three<br />
gametes at one time is prohibited, while cryopreservation<br />
of surplus oocytes is allowed by the Italian law 40/2004<br />
that regulates assisted reproductive technology<br />
(Benagiano and Gianaroli, 2004). All the patients<br />
undergoing an IVF treatment in these five assisted<br />
reproductive centres were included in a salvage oocyte<br />
cryopreservation programme by being offered the<br />
opportunity to have their surplus oocytes cryopreserved.<br />
The retrospective survey was carried out on 510 oocytethawing<br />
cycles performed between March 2004 and<br />
March 2008. The oocytes from 424 patients were<br />
cryopreserved; the mean age ± SE of the patients at the<br />
time of oocyte retrieval was 34.46 ± 0.17 years. All the<br />
women included in this study were informed about the<br />
procedure and written consent was obtained from each<br />
one. The procedures were approved by the Institutional<br />
Review Boards of each centre. Oocyte cryopreservation<br />
was usually carried out from 1 h up to several hours after<br />
oocyte retrieval. There was retrospective observation of<br />
the influence of freezing within 2 h or more than 2 h from<br />
oocyte retrieval on the out<strong>com</strong>e of the oocyte-thawing<br />
cycles. To this aim, the thawing cycles were divided into<br />
two groups: group A, oocytes were frozen within 2 h of<br />
retrieval; group B, oocytes were frozen more than 2 h after<br />
retrieval.<br />
CONTINUED ON PAGE 14<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
13
INTERNATIONAL NEWS<br />
CONTINUED FROM PAGE 13<br />
Ovarian stimulation, oocyte retrieval and selection<br />
Ovarian stimulation was achieved using<br />
gonadotrophin-releasing hormone analogues in<br />
<strong>com</strong>bination with a graded gonadotrophin administration.<br />
Transvaginal ultrasound-guided oocyte retrieval was<br />
performed 35.82 ± 0.02 h (mean ± SE; range 35–36 h) after<br />
ovulation induction with either 5000 or 10,000 IU of HCG,<br />
depending on the procedures of each assisted<br />
reproduction. After retrieval, oocytes were cultured for<br />
between 1 and 7 h at 37°C in an atmosphere of either 5% or<br />
6% CO2 before the <strong>com</strong>plete removal of cumulus mass and<br />
corona cells by enzymatic digestion of hyaluronidase, and<br />
by gentle mechanic aspiration with plastic denuding<br />
pipettes. The denuded oocytes were then evaluated to<br />
assess their nuclear maturation stage. The oocytes that had<br />
released the first polar body [metaphase II (MII)]<br />
underwent a strict selection by morphological features<br />
(zona pellucida thickness, perivitelline space size, oocyte<br />
shape, cytoplasm colour and granularity, presence of<br />
vacuoles and first polar body morphology) under an<br />
inverted microscope with Hoffman modulation contrast.<br />
The oocytes classified as ‘high quality’ were those which<br />
were colourless and of regular shape, with regular zona<br />
pellucida and small perivitelline space without debris,<br />
homogeneous cytoplasm and no vacuoles or granulations<br />
(De Sutter et al., 1996; Xia, 1997; Ebner et al., 2003).<br />
Amongst the ‘high quality’ oocytes, the presence of an<br />
intact, round or ovoid polar body with smooth surface was<br />
considered as a selection criterion (Ebner et al., 2000).<br />
Immediately after decumulation and quality evaluation,<br />
the three best available MII oocytes were inseminated by<br />
ICSI, according to the Italian law regulating assisted<br />
reproductive technology. Only the supernumerary MII<br />
oocytes reaching the ‘high quality’ standards were<br />
cryopreserved.<br />
Cryopreservation protocol<br />
The cryopreservation protocol consisted of a slowfreezing–rapid-thawing<br />
method. Oocyte freezing and<br />
thawing solutions (OocyteFreeze–OocyteThaw;<br />
MediCult, Jyllinge, Denmark) contained Dulbecco’s<br />
phosphate-buffered saline (PBS) supplemented with<br />
human serum albumin, α- and β-globulins, and 1,2-PROH<br />
and sucrose as cryoprotectants.<br />
Freezing procedure<br />
After washing in a PBS solution (vial 1,<br />
OocyteFreeze; MediCult), the oocytes were equilibrated<br />
for 10 min at room temperature in 1.5 mol/l 1,2-PROH<br />
(vial 2, OocyteFreeze; MediCult) and then transferred<br />
into the loading solution of 1.5 mol/l 1,2-PROH and 0.3<br />
mol/l sucrose (vial 3, OocyteFreeze; MediCult). Between<br />
one and three oocytes were loaded in plastic straws<br />
(Paillette Cristal 133 mm; Cryo Bio System, Paris, France)<br />
and transferred into an automated biological vertical<br />
freezer (Kryo 360-1.7; Planer, Sunbury, UK). The cooling<br />
process was initiated reducing chamber temperature from<br />
20°C to –7°C at a rate of 2°C/min. Ice nucleation was<br />
induced manually at –7°C. After a hold time of 10 min at<br />
–7°C, the straws were cooled slowly to –30°C at a rate of<br />
0.3°C/min and then rapidly to –150°C at a rate of<br />
50°C/min. After 10–<strong>12</strong> min at stabilization temperature,<br />
the straws were transferred into liquid nitrogen and<br />
stored for later use.<br />
Thawing procedure<br />
The straws were air-warmed for 30 s and then<br />
immersed in a 30°C water bath for 40 s. The cryoprotectant<br />
was removed at room temperature by stepwise dilution of<br />
1,2-PROH in the thawing solutions: the contents of the<br />
straws were expelled in 1.0 mol/l 1,2-PROH and 0.3 mol/l<br />
sucrose solution (vial 1, OocyteThaw; MediCult) and<br />
the oocytes were equilibrated for 5 min. The oocytes were<br />
then transferred into 0.5 mol/l 1,2-PROH and 0.3 mol/l<br />
sucrose solution (vial 2, OocyteThaw; MediCult) for 5<br />
min and then into 0.3 mol/l sucrose solution (vial 3,<br />
OocyteThaw; MediCult) for 10 min before final dilution<br />
in PBS solution (vial 4, OocyteThawTM; MediCult) for<br />
20 min (10 min at room temperature and 10 min at 37°C).<br />
The oocytes were finally cultured at 37°C in an<br />
atmosphere of 5% or 6% CO2 in air for 2.79 ± 0.05 h (range<br />
2–5 h) before ICSI.<br />
Survival evaluation, ICSI and embryo culture<br />
After the post-thaw culture the three best surviving<br />
oocytes, according to the previously described<br />
parameters, were inseminated by ICSI, as allowed under<br />
the Italian IVF act. The evaluation of survival was carried<br />
out by inverted microscope with Hoffman modulation<br />
contrast, and thawed oocytes were considered to have<br />
survived in the absence of negative characteristics: dark or<br />
contracted ooplasm, vacuolization, cytoplasmic leakage,<br />
abnormal perivitelline space, cracked zona pellucida. The<br />
surviving oocytes were selected prior to ICSI following<br />
the centre’s own ‘high quality’ standards. Amongst the<br />
14 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
INTERNATIONAL NEWS<br />
‘high quality’ thawed oocytes, the presence of an intact<br />
polar body was considered as a selection criterion; as far as<br />
possible, injecting thawed oocytes presenting an atretic<br />
polar body or a so-called ‘ghost polar body’, which is an<br />
empty membrane without cytoplasm, was avoided (La<br />
Sala et al., 2006). Fertilization and embryo development<br />
were examined by inverted microscope. Embryos were<br />
graded 1–5 (1 best, 5 worst), with grade 1 assigned to the<br />
best quality embryos containing equally sized<br />
symmetrical blastomeres with no fragmentation,<br />
according to the criteria previously described by Veeck<br />
(1999). The embryo development rating (EDR) as<br />
described by Cummins et al. (1986) was calculated to<br />
define the growth rate of transferred embryos obtained by<br />
thawed oocytes. The formula for calculating the EDR was<br />
as follows: EDR = (TE/TO) × 100 (TE = time expected, TO =<br />
time observed). The ideal EDR is 100: this value is obtained<br />
when a hypothetical ‘normally’ growing embryo is at the<br />
2-cell stage at 33.6 h, at the 4-cell stage at 45.5 h, and at 8-<br />
cell stage at 56.4 h.<br />
Endometrial preparation and embryo transfer<br />
Preparation of the endometrium for the embryo<br />
transfer involved the natural ovulatory cycle or a hormone<br />
replacement cycle. An endometrial thickness .8 mm was<br />
considered to be optimal for performing an embryo<br />
transfer. Embryo transfer was carried out after 2 (day 2) or<br />
3 days (day 3) from oocyte thawing and ICSI. In one case<br />
(group B), the embryo transfer was carried out after 6 days<br />
(day 6 blastocyst stage) from thawing and ICSI; testing for<br />
HCG was performed 14 days after embryo transfer.<br />
Clinical pregnancy was defined as the presence of a<br />
gestational sac with or without fetal heart beat at<br />
ultrasound examination 2 weeks after positive HCG<br />
testing.<br />
Statistical analysis<br />
Continuous variables are presented as means ± SE.<br />
Categorical variables are presented as percentages.<br />
Normality of distribution of continuous variables was<br />
assessed with a Kolmogorov–Smirnov test (with Lillefor<br />
correction). Between-group differences of normally<br />
distributed continuous variables were assessed with<br />
parametric statistic (Student’s t-test), whereas nonparametric<br />
statistics (Mann.Whitney Rank Sum Test) were<br />
employed when the normality test was not passed.<br />
Between-group differences in non-continuous variables<br />
were assessed using the chi-squared method with Yates<br />
correction if needed, or with Fisher’s exact test. A<br />
difference was considered significant when a P-value was<br />
INTERNATIONAL NEWS<br />
CONTINUED FROM PAGE 15<br />
16 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
INTERNATIONAL NEWS<br />
methods). A total of 1139 oocytes fertilized after ICSI<br />
(fertilization rate: 82.60%) and 1037 cleaved (cleavage rate:<br />
91.04%). Oocyte survival, fertilization and cleavage were<br />
<strong>com</strong>parable in all the groups (Table 3). Embryo<br />
development rating (EDR) was significantly higher (85.54<br />
± 1.00) in group A than in group B (83.66 ± 0.85, P = 0.047).<br />
The best quality embryo rate (grade 1) in group A was<br />
significantly higher (33.24%) than in group B (16.20%, P <<br />
0.001) and in total (14.1%, P = 0.002).<br />
Discussion<br />
Initial studies on human oocyte cryopreservation<br />
(Chen, 1986; Al-Hasani et al., 1987) were mainly conducted<br />
using a slow-freezing/rapid-thawing method (SF/RT)<br />
based on criteria optimized for embryo freezing (Trounson<br />
and Mohr, 1983; Lassalle et al., 1985). The original SF/RT<br />
methodology underwent various modifications (Gook et<br />
al., 1993, 1994, 1995a,b), such as the introduction of<br />
elevated dehydrating sucrose concentrations (Yang et al.,<br />
1998; Fabbri et al., 2001; Bianchi et al., 2007), designed to<br />
improve oocyte survival and clinical results (Winslow et<br />
al., 2001; Yang et al., 2002; Fosas et al., 2003; Chen et al.,<br />
2005; Li et al., 2005; Borini et al., 2006b). The optimal time<br />
for ICSI of a SF/RT oocyte is strictly dependent on the<br />
<strong>com</strong>plete restoration of cellular function and in particular<br />
the organization of the meiotic spindle; a post-thaw<br />
incubation of approximately 3 h is re<strong>com</strong>mended to allow<br />
spindle reappearance in slow frozen oocytes (Rienzi et al.,<br />
2004; Bianchi et al., 2005). The timing of ICSI can affect<br />
embryo implantation: even though it is possible to achieve<br />
implantation of embryos derived from aged oocytes (Chen<br />
and Kattera, 2003), it has been shown that the<br />
developmental capacity of the oocyte declines 10 h after<br />
retrieval (Yanagida et al., 1998). Insemination of fresh<br />
oocytes between 37 and 41 h after HCG administration to<br />
trigger ovulation determines the highest embryo<br />
implantation rate (Dozortsev et al., 2004). Therefore, the<br />
metabolic ageing at ICSI of slow-cooled oocytes depends<br />
on: (i) time of retrieval after HCG administration; (ii) preincubation<br />
before cryopreservation; and (iii) post-thawing<br />
culture before insemination (Parmegiani et al., 2008a). In<br />
this multicentre study, oocyte retrieval was performed<br />
35.82 ± 0.02 h (range 35.36 h) after HCG administration<br />
and thawed oocytes were injected after 2.79 ± 0.05 h (range<br />
2–5 h) of post-thaw culture. Thus, the total timing of<br />
insemination (i + ii + iii) was (35.82 h + ≤2 h + 2.79 h) for<br />
oocytes in group A, and (35.82 h+ >2 h + 2.79 h) for those<br />
in group B. When using a SF/RT protocol, the time of<br />
incubation between oocyte retrieval and cryopreservation<br />
is critical in order to avoid injecting ‘aged’ oocytes. In this<br />
survey, ICSI was performed with ideal timing (
INTERNATIONAL NEWS<br />
CONTINUED FROM PAGE 17<br />
Regarding the preservation of the cellular function<br />
(fertilization, cleavage and implantation) the results of this<br />
study were at least <strong>com</strong>parable with the other studies<br />
using SF/RT with high sucrose concentration (Gook and<br />
Edgar, 2007).Fertilization and cleavage rates did not<br />
significantly vary among the study groups, even though<br />
the embryo quality was influenced by the time of freezing:<br />
a significant increase of top quality embryo rate by<br />
freezing the oocytes within 2 h from oocyte retrieval rather<br />
than freezing them after 2 h was observed (Table 3). It was<br />
observed that the growth rate of embryos (EDR) obtained<br />
from thawed oocytes (84.31 ± 0.65) was lower than the<br />
hypothetical ‘normally’ growing embryo rate (100)<br />
theorized by Cummins et al. (1986). This retarded<br />
development of embryos obtained fro m oocytes<br />
cryopreserved in 0.3 mol/l sucrose has already been<br />
reported by Parmegiani et al. (2008a) in the previous study<br />
on SF/RT 0.3. Nevertheless, in the present multicentre<br />
survey, a significantly higher mean EDR was seen in group<br />
A than in group B confirming the positive effect of timely<br />
cryopreservation (Table 3).<br />
Efficiency of oocyte freezing can be measured as<br />
implantation and pregnancy rates obtained per oocytes<br />
thawed or per embryos transferred (Oktay et al., 2006). The<br />
number of oocytes frozen and the number of thawing<br />
cycles performed vary widely among published reports on<br />
oocyte cryopreservation. Highest implantations per oocyte<br />
thawed were reported in thawing cycles of SF/RT high<br />
sucrose cryopreserved donor oocytes: 21/158 (13.3%) by<br />
Yang et al. (2002), and 10/81 (<strong>12</strong>.3%) by Li et al. (2005). The<br />
best clinical results in larger studies on SF/RT (Porcu et al.,<br />
2000, Borini et al. 2006a,b; La Sala et al., 2006; Levi Setti et<br />
al., 2006; De Santis et al., 2007) were reported by Bianchi et<br />
al. (2007), with implantation and pregnancy rates per<br />
transfer of 13% and 21% respectively, resulting in 6.0%<br />
(24/403) of implantation rate per thawed oocyte. In the<br />
present multi-centre study, the overall pregnancy rate per<br />
transfer reported was 16.25%: 72 clinical pregnancies were<br />
obtained after 443 embryo transfers, with 85 implantations<br />
from 1037 transferred embryos (8.20% of implantation<br />
rate) (Table 1) and from 2608 thawed oocytes (3.26%)<br />
(Table 2). The data are <strong>com</strong>parable with the results<br />
obtained in the wider studies on SF/RT. A high miscarriage<br />
rate (32%) was observed in this study, especially when<br />
<strong>com</strong>pared with the miscarriages observed in fresh cycles<br />
(about 22%) in the five centres involved in this multicentre<br />
survey in the same period as the study (March 2004 to<br />
March 2008). Nevertheless, the miscarriage rate observed<br />
in this study is lower than that reported by La Sala et al.<br />
(2006) in a wide study on SF/RT 0.3 (performed under the<br />
same legal restrictions and using the same SF/RT 0.3<br />
protocol) involving 518 cryopreservation treatments. In<br />
fact, the authors of that study reported a miscarriage rate<br />
of 47% and a take-home baby rate/embryo transfer of 1.5%<br />
(7/456) resulting in a probability of one live birth in 65<br />
embryo transfers. The results on salvaging oocyte<br />
cryopreservation observed in the present multicentre<br />
survey can be considered more encouraging: there was a<br />
take-home baby rate/embryo transfer of 9.7% (43/443)<br />
with a probability of one live birth in 10 embryo transfers.<br />
Furthermore, the lowest miscarriage rate observed in this<br />
study for group A (28%) suggests a better trend with<br />
oocytes frozen within 2 h.<br />
In this multicentre study, observing the results in<br />
group A, in which thawing cycles were performed with<br />
oocytes previously frozen within 2 h from oocyte retrieval,<br />
pregnancy and implantation rates were 30.07% and<br />
15.08%, with 6.49 implantations per 100 oocytes thawed.<br />
The pregnancy and implantation rates per transfer and<br />
per thawed–injected oocytes observed in group A were all<br />
significantly higher than in group B, in which oocytes<br />
were frozen after more than 2 h of pre-incubation. The<br />
better efficiency rates observed when oocytes were frozen<br />
within 2 h from oocyte retrieval confirmed, on a larger<br />
number of thawing cycles, the preliminary observation of<br />
Parmegiani et al. (2008a), which reported the highest<br />
implantation rate per oocytes thawed (8.1) in studies<br />
regarding homologous SF/RT.<br />
A very promising alternative to the slow-cooling<br />
protocol is vitrification, in which the gametes, immersed<br />
in a viscous solution with a high concentration of<br />
cryoprotectants, are cooled at an extremely rapid rate. In<br />
the wider studies with vitrification protocols, an<br />
implantation rate per thawed oocyte of 11.2% (<strong>12</strong>/107) was<br />
reported by Kuwayama et al. (2005), and 11.8% (39/330) by<br />
Antinori et al. (2007). It was encouraging that the results of<br />
this multicentre survey suggest that freezing oocytes via<br />
SF/RT 0.3 within 2 h allows optimal clinical results to be<br />
achieved that are almost <strong>com</strong>parable with those obtained<br />
with vitrification.<br />
In conclusion, this multicentre study confirmed on a<br />
large scale the fact that the efficiency of oocyte SF/RT 0.3 is<br />
improved if the freezing procedure is carried out within 2<br />
h of oocyte retrieval. In fact, embryo quality and EDR,<br />
clinical pregnancies and implantations per transfer and<br />
per oocytes thawed/injected, were all significantly<br />
increased when oocytes were frozen within 2 h of<br />
retrieval. Thus, it is concluded that freezing within 2 h of<br />
retrieval optimizes oocyte cryopreservation when using<br />
SF/RT 0.3.<br />
18 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
INTERNATIONAL NEWS<br />
Acknowledgements<br />
The authors wish to thank Ms Maggie Baigent for<br />
revising the manuscript. The authors also wish to thank<br />
Mr Giovanni Ermini (MediCult, Italy), Ms Susanne<br />
Hauschildt Bendz (MediCult, Denmark) and Ms Francesca<br />
Granella (Livet Clinic, Turin, Italy) for their valuable<br />
collaboration in the collection of the data.<br />
References<br />
Al-Hasani S, Diedrich K, Van der Ven H et al. 1987<br />
Cryopreservation of human oocytes. Human Reproduction 2,<br />
695–700.<br />
Antinori M, Licata E, Dani G et al. 2007 Cryotop vitrification of<br />
human oocytes results in high survival rate and healthy<br />
deliveries. Reproductive BioMedicine Online 14, 72–79.<br />
Benagiano G, Gianaroli L 2004 The new Italian IVF legislation.<br />
Reproductive BioMedicine Online 9, 117–<strong>12</strong>5.<br />
Bianchi V, Coticchio G, Distratis V et al. 2007 Differential sucrose<br />
concentration during dehydration (0.2 mol/l) and<br />
rehydration (0.3 mol/l) increases the implantation rate of<br />
frozen human oocytes. Reproductive BioMedicine Online 14,<br />
64–71.<br />
Bianchi V, Coticchio G, Fava L et al. 2005 Meiotic spindle imaging<br />
in human oocytes frozen with a slow freezing procedure<br />
involving high sucrose concentration. Human Reproduction<br />
20, 1078–1083.<br />
Borini A, Lagalla C, Bonu MA et al. 2006a Cumulative pregnancy<br />
rates resulting from the use of fresh and frozen oocytes: 7<br />
years’ experience. Reproductive BioMedicine Online <strong>12</strong>,<br />
481–486.<br />
Borini A, Sciajno R, Bianchi V et al. 2006b Clinical out<strong>com</strong>e of<br />
oocyte cryopreservation after slow cooling with a protocol<br />
utilizing a high sucrose concentration. Human Reproduction<br />
21, 5<strong>12</strong>–517.<br />
Chamayou S, Alecci C, Ragolia C et al. 2006 Comparison of invitro<br />
out<strong>com</strong>es from cryopreserved oocytes and sibling fresh<br />
oocytes. Reproductive BioMedicine Online <strong>12</strong>, 730–736.<br />
Chen C 1986 Pregnancy after human oocyte cryopreservation.<br />
Lancet 1, 884–886.<br />
Chen C, Kattera S 2003 Rescue ICSI of oocytes that failed to<br />
extrude the second polar body 6h post-insemination in<br />
conventional IVF. Human Reproduction 18, 2118–2<strong>12</strong>1.<br />
Chen SU, Lien YR, Chen HF et al. 2005 Observational clinical<br />
follow-up of oocyte cryopreservation using a slow freezing<br />
method with 1,2-propanediol plus sucrose followed by ICSI.<br />
Human Reproduction 20, 1975–1980.<br />
Cummins JM, Breen TM, Harrison KL et al. 1986 A formula for<br />
scoring human embryo growth rates in in vitro fertilization:<br />
its value in predicting pregnancy and in <strong>com</strong>parison with<br />
visual estimates of embryo quality. Journal of In Vitro<br />
Fertilization and Embryo Transfer 3, 284–295.<br />
De Santis L, Cino I, Coticchio G et al. 2007 Objective evaluation of<br />
the viability of cryopreserved oocytes. Reproductive<br />
BioMedicine Online 15, 338–345.<br />
De Sutter P, Dozortsev D, Qian C et al. 1996 Oocyte morphology<br />
does not correlate with fertilization rate and embryo quality<br />
after intracytoplasmic sperm injection. Human Reproduction<br />
11, 595–597.<br />
Dozortsev D, Nagy P, Abdelmassih S et al. 2004 The optimal time<br />
for intracytoplasmic sperm injection in the human is from 37<br />
to 41 h after administration of human chorionic<br />
gonadotropin. Fertility and Sterility 82, 1492–1496.<br />
Ebner T, Moser M, Sommergruber M et al. 2003 Selection based<br />
on morphological assessment of oocytes and embryos at<br />
different stages of preimplantation development: a review.<br />
Human Reproduction Update 9, 251–262.<br />
Ebner T, Yaman C, Moser M et al. 2000 Prognostic value of first<br />
polar body morphology on fertilization rate and embryo<br />
quality in intracytoplasmic sperm injection. Human<br />
Reproduction 15, 427–430.<br />
Fabbri R, Porcu E, Marsella T et al. 2001 Human oocyte<br />
cryopreservation: new perspectives regarding oocyte<br />
survival. Human Reproduction 16, 411–416.<br />
Fosas N, Marina F, Torres FJ et al. 2003 The births of five Spanish<br />
babies from cryopreserved donated oocytes. Human<br />
Reproduction 18, 1417–1421.<br />
Gook DA, Edgar DH 2007 Human ooocyte cryopreservation.<br />
Human Reproduction Update 6, 591–605.<br />
Gook DA, Osborn SM, Johnston WI 1995a Parthenogenetic<br />
activation of human oocytes following cryopreservation<br />
using 1,2-propanediol. Human Reproduction 10, 654–658.<br />
Gook DA, Schievwe MC, Osborn SM et al. 1995b<br />
Intracytoplasmic sperm injection and embryo development<br />
of human oocytes cryopreserved using 1,2-propanediol.<br />
Human Reproduction 10, 2637–2641.<br />
Gook DA, Osborn SM, Bourne H et al. 1994 Fertilization of<br />
human oocytes following cryopreservation; normal<br />
karyotypes and absence of stray chromosomes. Human<br />
Reproduction 9, 684–691.<br />
Gook DA, Osborn SM, Johnston WI 1993 Cryopreservation of<br />
mouse and human oocytes using 1,2-propanediol and the<br />
configuration of the meiotic spindle. Human Reproduction 8,<br />
1101–1119.<br />
Hunter JE, Bernard A, Fuller BJ et al. 1992 Measurement of the<br />
membrane water permeability (Lp) and its temperature<br />
dependence (activation energy) in human fresh and failed to<br />
fertilize oocytes and mouse oocytes. Cryobiology 29, 240–249.<br />
Jain JK, Paulson RJ 2006 Oocyte cryopreservation. Fertility and<br />
Sterility 86, 1037–1046.<br />
Kuleshova L, Gianaroli L, Magli C et al. 1999 Birth following<br />
vitrification of a small number of human oocytes: case<br />
report. Human Reproduction 14, 3077–3079<br />
Kuwayama M, Vaita G, Kato O et al. 2005 Highly efficient<br />
vitrification method for cryopreservation of human oocytes.<br />
Reproductive BioMedicine Online 11, 300–308.<br />
La Sala GB, Vicoli A, Villani MT et al. 2006 Out<strong>com</strong>e of 518<br />
salvage oocyte-cryopreservation cycles performed as a<br />
routine procedure in an in vitro fertilization program.<br />
Fertility and Sterility 86, 1423–1427.<br />
Lassalle B, Testart J, Renard JP 1985 Human embryo features that<br />
influence the success of cryopreservation with the use of 1,2-<br />
propanediol. Fertility and Sterility 44, 645–651.<br />
CONTINUED ON PAGE 20<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
19
INTERNATIONAL NEWS<br />
CONTINUED FROM PAGE 19<br />
Levi Setti PE, Albani E, Novara PV et al. 2006 Cryopreservation of<br />
supernumerary oocytes in IVF/ICSI cycles. Human<br />
Reproduction 21, 370–375.<br />
Li XH, Chen SU, Zhang X et al. 2005 Cryopreserved oocytes of<br />
infertile couples undergoing assisted reproductive<br />
technology could be an important source of oocytes<br />
donation: a clinical report. Human Reproduction 20,<br />
3390–3394.<br />
Oktay K, Cil AP, Bang H 2006 Efficiency of oocyte<br />
cryopreservation: a meta analysis. Fertility and Sterility 86,<br />
70–80.<br />
Parmegiani L, Cognigni GE, Bernardi S et al. 2008a Freezing<br />
within 2 h from oocyte retrieval increases the efficiency of<br />
human oocyte cryopreservation when using a slow<br />
freezing/rapid thawing protocol with high sucrose<br />
concentration. Human Reproduction 23, 1771–1777.<br />
Parmegiani L, Fabbri R, Cognigni GE et al. 2008b Blastocyst<br />
formation, pregnancy, and birth derived from human<br />
oocytes cryopreserved for 5 years. Fertility and Sterility 90,<br />
2014.e7–10.<br />
Porcu E, Fabbri R, Marsella T et al. 2000 Clinical experience and<br />
application of oocyte cryopreservation. Molecular and<br />
Cellular Endocrinology 169, 33–37.<br />
Porcu E, Fabbri R, Seracchioli R et al. 1997 Birth of a healthy<br />
female after intracytoplasmic sperm injection of<br />
cryopreserved human oocytes. Fertility and Sterility 68,<br />
724–726.<br />
Practice Committee of the American Society for Reproductive<br />
Medicine 2006 Ovarian tissue and oocyte cryopreservation.<br />
Fertility and Sterility 86, S142–S147<br />
Rienzi L, Martinez F, Ubaldi F et al. 2004 PolScope analysis of<br />
meiotic spindle changes in living metaphase II oocytes<br />
during the freezing and thawing procedures. Human<br />
Reproduction 19, 655–659.<br />
Trounson A, Mohr L 1983 Human pregnancy following<br />
cryopreservation, thawing and transfer of an eight-cell<br />
embryo. Nature 305, 707–709.<br />
Veeck LL 1999 Pre-embryo grading and degree of cytoplasmic<br />
fragmentation. In: Veeck LL (ed.) An Atlas of Human Gametes<br />
and Conceptuses. Parthenon, New York pp. 40–45.<br />
Winslow K, Yang D, Blohm P et al. 2001 Oocyte<br />
cryopreservation/a three year follow up of sixteen births.<br />
Fertility and Sterility 76 (Suppl. 1), S<strong>12</strong>0–S<strong>12</strong>1.<br />
Xia P 1997 Intracytoplasmic sperm injection: correlation of<br />
oocyte grade based polar body, perivitelline space and<br />
cytoplasmic inclusion with fertilization rate and embryo<br />
quality. Human Reproduction <strong>12</strong>, 1750–1755.<br />
Yanagida K, Yazawa H, Katayose H et al. 1998 Influence of oocyte<br />
preincubation time on fertilization after intracytoplasmic<br />
sperm injection. Human Reproduction 13, 2223–2226.<br />
Yang D, Brown SE, Nguyen K, et al. 2007 Live birth after the<br />
transfer of human embryos developed from cryopreserved<br />
oocytes harvested before cancer treatment. Fertility and<br />
Sterility 87, 1469.e1–4.<br />
Yang D, Winslow K, Blohm P et al. 2002 Oocyte donation using<br />
cryopreserved donor oocytes. Fertility and Sterility 78 (Suppl.<br />
1), S14.<br />
Yang D, Blohm P, Winslow K et al. 1998 A twin pregnancy after a<br />
microinjection of human cryopreserved oocyte with a<br />
specially developed oocyte cryopreservation regime.<br />
Fertility and Sterility 70 (Suppl. 1), S239.<br />
20 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
The 3 most reliable<br />
Aspiration Pumps for IVF…<br />
Pioneer IVF has provided the most reliable Pro-Pump series available in IVF, the single<br />
vacuum, the dual vacuum and the TriVac model. Pioneer Pro-Pumps are used in a majority<br />
of IVF laboratories worldwide and are available in both 115V and 230V.<br />
Single Vac Pioneer Pro-Pump<br />
This standard aspiration pump is a powerful, durable Pioneer Pro-<br />
Pump operated with a single foot pedal. A fully adjustable<br />
vacuum control knob provides an even vacuum flow from 0-450<br />
mmHg. Switch to ‘high’ with the push of a button on the front<br />
panel.<br />
Pro-Pump Single (115V) . . .GPPS-010/115<br />
Pro-Pump Single (230V) . . .GPPS-010/230<br />
Accessory Kit . . . . . . . . . . . .GPPK-075<br />
Dual Vac Pioneer Pro-Pump<br />
The dual vacuum model lets you set a ‘low’ vacuum level and<br />
switch to a ‘high’ vacuum level simply by pressing the foot pedal.<br />
A fully adjustable vacuum control knob provides an even vacuum<br />
flow from 0-450 mmHg.<br />
Pro-Pump Dual (115V) . . . . .GPPD-050/115<br />
Pro-Pump Dual (230V) . . . .GPPD-050/230<br />
Accessory Kit . . . . . . . . . . . .GPPK-075<br />
TriVac Pro-Pump<br />
The TriVac lets you set a Level 1, Safe ‘low’ level and a second<br />
Level 2, Safe ‘mid’ range which is activated by the foot pedel. It<br />
lets you set Level 1 at a very safe 40-80 mmHg vacuum and<br />
switch to Level 2 at 80-100 mmHg vacuum with your foot pedal.<br />
It gives you a Level 3 ‘high’ vacuum with the push of a button on<br />
the front panel.<br />
Pioneer TriVac (115V) . . . . .PTPP-010/115<br />
Pioneer TriVac (230V) . . . . .PTPP-010/230<br />
Accessory Kit . . . . . . . . . . . .PTAK-010<br />
Patent Pending<br />
Pioneer Pro-Pumps are the most reliable aspiration pumps used in IVF.<br />
• Quietest in the Industry • Responsive • Steel Housing • ISO 13485:2003<br />
• Light Weight • 5 Year Warranty • 115V & 230V • ISO 9001:2000<br />
• Vacuum 0-300 mmHg • Made in USA • CE Registered • FDA 510(k) Cleared<br />
www.IVFonline.<strong>com</strong>
ARTICLES<br />
Effect of growth hormone on oocyte <strong>com</strong>petence in<br />
patients with multiple IVF failures<br />
A Hazout 1,3 , AM Junca1, Y Ménézo 1 , J de Mouzon 2 , P Cohen-Bacrie 1<br />
1 ART Unit, EYLAU, 55 Rue Saint Didier, 75016 Paris, France; 2 Unité, INSERM, U822 Kremlin-Bicêtre, France<br />
3 Correspondence: e-mail: ahazout@hotmail.<strong>com</strong><br />
c 2010, Reproductive Healthcare Ltd. Published by Elsevier Ltd. All rights reserved.<br />
Dr. André Hazout, 2009 Effect of growth hormone on oocyte <strong>com</strong>petence in patients with multiple IVF failures. Reprod BioMed Online,<br />
18, 5, 664-670.<br />
André Hazout was co-leader of the assisted reproduction program of Dr Frydman’s<br />
team in Clamart from 1983 to 2003 and leader of the private assisted reproduction<br />
unit ‘Eylau la Muette’ until 2008. From 2003 to 2008 he also led the assisted<br />
reproduction program of the University Paris VII. His recent research interests<br />
include male infertility, sclerotherapy of endometriosis cysts and embryo<br />
implantation markers in endothelium explants. Throughout his career he has been<br />
responsible for many initiatives and has also published extensively in both national<br />
and international journals. He is a past President of the French Society of<br />
Reproductive Medicine.<br />
DR ANDRÉ HAZOUT<br />
Abstract<br />
In a preliminary, unpublished randomized study conducted in 2000 on 39 patients, including a placebo group, it was observed that<br />
the addition of growth hormone (GH) during ovarian stimulation in patients with poor-quality oocytes increased the pregnancy<br />
rate. However, the results were not statistically significant due to the small number of patients in each group. A protocol with 8 IU<br />
GH was tested in 291 patients with three or more previous failures of embryo transfer for no clearly identifiable reasons. The<br />
analysis was restricted to patients receiving either re<strong>com</strong>binant FSH or human menopausal gonadotrophin (HMG) (n = 245). They<br />
were <strong>com</strong>pared retrospectively to all patients with three or more failures during the same period of time but stimulated only with<br />
re<strong>com</strong>binant FSH or HMG, without GH, in an observational study design. Co-stimulation with GH gave better results in terms of<br />
number of oocytes collected and embryos obtained. Pregnancy rate per retrieval was higher than in the control group (25.7%<br />
versus 18.2%, P < 0.01) and reached a level similar to the one observed in the study centre for the whole population. Ovarian<br />
stimulation associated with GH can be proposed for patients with a history of repeated assisted reproduction failures. An<br />
improvement of cytoplasmic <strong>com</strong>petence is proposed as an explanation.<br />
Keywords: growth hormone, ICSI, IVF, ovarian stimulation<br />
Introduction<br />
Recurrent IVF failure is always a source of distress in<br />
patients, especially in the group of so-called normoresponders<br />
where ovarian stimulation is expected to give<br />
acceptable results. The oocytes of this group of patients<br />
are often classified as dysmorphic (Van Blerkom and<br />
Henry, 1992); this includes abnormal aspects of cytoplasm,<br />
perivitelline space and zona pellucida. Early embryo<br />
preimplantation development is under maternal control<br />
until maternal to zygotic transition. The first cleavages are<br />
under control of mRNA and proteins stored during<br />
maturation; the quality of these stores is directly related to<br />
the cytoplasmic maturation, i.e. <strong>com</strong>petence in order to<br />
22 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
allow early preimplantation development in a correct<br />
timing. Even if the pivotal role of gonadotrophins cannot<br />
be underestimated, other co-effectors such as insulin-like<br />
growth factor-I (IGF-I), leukaemia inhibitory factor (LIF)<br />
and growth hormone (GH) cannot be neglected especially<br />
for their role in enhancing cytoplasmic maturation (Ptak et<br />
al., 2006; De Matos et al. 2008). In cultured cow (Langhout<br />
et al., 1991) and human (Mason et al., 1990), GH stimulates<br />
steroidogenesis either directly or through enhancement of<br />
the effect of FSH. The positive impact of GH, i.e. the<br />
improvement of nuclear and cytoplasmic maturation,<br />
acting independently of IGF-I, is well documented in<br />
mouse, cow and monkey (Izadyar et al., 1996, 1998, 2000;<br />
Pantaleon et al., 1997; Modina et al., 2007, de Prada and<br />
VandeVoort, 2008). GH allows a full maturation of naked<br />
oocytes in humans (Hassan et al., 2001; Ménézo et al.,<br />
2006). The GH receptor is present in cumulus cells and in<br />
the oocyte for all these animal species as well as in humans<br />
(Ménézo et al., 2003). GH signalling is two-fold: it uses the<br />
signal transducer and activator of transcription (STAT) or<br />
the cAMP response element-binding (CREB), mitogenactivated<br />
protein (MAP) kinase pathways (Izadyar et al.,<br />
1999). Studies evaluating the benefit of co-stimulation<br />
treatment with GH have given discordant results<br />
(Schoolcraft et al., 1997; Howles et al., 1999) for a variety of<br />
aetiologies such as aged patients (Tesarik et al., 2004), poor<br />
responders and patients having GH deficiency (Rajesh et<br />
al., 2007).<br />
The most recently updated Cochrane database<br />
(Harper et al., 2003) showed a positive effect of GH in<br />
poor-responder patients (odds ratio [OR] 4.37, 95%<br />
confidence interval [CI] 1.06–18.01). The reviewer’s<br />
conclusion, based on the large CI and high upper value of<br />
18.01 and the fact that the database was from three rather<br />
small studies, was that ‘before re<strong>com</strong>mending GH in IVF,<br />
further research is necessary to fully define its role.<br />
Meanwhile GH should only be considered in the context<br />
of a clinical trial’. The aim of the current study was to<br />
investigate the effect of GH co-stimulation in ovarian<br />
stimulation in normo-responder patients, who had<br />
previously had at least three failed IVF/intracytoplasmic<br />
sperm injection (ICSI) cycles. In a preliminary,<br />
unpublished randomized study involving 39 patients,<br />
including a placebo group, conducted in 2000, it was<br />
observed that the addition of GH during ovarian<br />
stimulation in patients with poor-quality oocytes<br />
increased the pregnancy rate (50% in the arm with 4 IU<br />
GH daily [n = 13], 55% in the arm with 8 IU GH daily [n =<br />
13] and 18% in the placebo group [n = 13]). The dose of GH<br />
used in the current study was based on this previous<br />
investigation.<br />
Materials and methods<br />
An open, non-<strong>com</strong>parative and non-randomized<br />
study on the effect of the addition of re<strong>com</strong>binant GH (r-<br />
GH, Saizen ® ; Serono, Lyon, France) to gonadotrophins on<br />
assisted reproduction treatment out<strong>com</strong>e was performed<br />
in voluntary patients enrolled in the study centre, Clinique<br />
de la Muette (Paris, France) collaborating with the IVF<br />
laboratory (Laboratoire d’Eylau, Paris, France), between<br />
January 2002 and September 2007. The study was given<br />
approval by the ethical <strong>com</strong>mittee of the Assisted<br />
Reproduction Unit, Eylau Laboratory and by the official<br />
ethical <strong>com</strong>mittee of Comité Consultatif de Protection des<br />
Personnes en Recherche Biomédicale of Saint Germain en<br />
Laye Hospital. The patients received information on the<br />
protocol and they signed an informed consent form.<br />
Growth hormone group<br />
Inclusion criteria<br />
Patients were eligible if they met the following criteria:<br />
(i) at least three previous assisted cycle failures; (ii) regular<br />
spontaneous menstrual cycles of 25–30 days; (iii) FSH, LH,<br />
oestradiol, inhibin B and anti-Müllerian hormone<br />
concentrations in the normal range during the early<br />
follicular phase; (iv) unexplained infertility with normal<br />
spermatozoa before IVF, or subnormal spermatozoa<br />
justifying ICSI; (v) less than 50% of dysmorphic oocytes in<br />
their previous assisted cycles; (vi) no treatment with<br />
gonadotrophins within 1 month of the treated cycle for the<br />
study; (vii) normal uterine cavity; (viii) negative<br />
pregnancy test; (ix) willingness to participate and to<br />
<strong>com</strong>ply with the protocol.<br />
Exclusion criteria<br />
Exclusion criteria were the following: abnormal<br />
gynaecological bleeding of undetermined origin,<br />
gonadotrophin allergy, known evolutive tumour, any<br />
unstabilized chronic pathology, medical treatments with<br />
corticoids, known human immunodeficiency virus and<br />
hepatitis B and C positive serology (according to the<br />
French legislation) or inability to <strong>com</strong>ply with the<br />
protocol.<br />
Stimulation protocol<br />
Starting on day 20 of the previous cycle, patients<br />
received daily injections of gonadotrophin-releasing<br />
CONTINUED ON PAGE 24<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
23
ARTICLES<br />
CONTINUED FROM PAGE 23<br />
hormone agonist (GnRHa, Decapeptyl ® ; 0.1 mg, Ipsen<br />
Laboratory France). The daily dose was decreased to 0.05<br />
mg after the confirmation of down-regulation and this<br />
reduced dose was maintained until the day of ovulation<br />
induction with 10,000 IU urinary human chorionic<br />
gonadotrophin (HCG; Gonadotrophine chorionique<br />
endo ® , Organon, France). Pituitary down-regulation was<br />
confirmed by an ultrasound scan showing no evidence of<br />
ovarian activity and/or serum concentrations of oestradiol<br />
16 mm in diameter and<br />
an oestradiol concentration of 140 pg/ml per follicle) were<br />
reached. An injection of HCG was given within 24 h of the<br />
last gonadotrophin administration. Oocytes were<br />
retrieved 36 h after HCG administration. Assessments of<br />
oocytes, 2 pronucleate zygotes and embryos were made on<br />
days 1, 2 and 3 after retrieval. Embryos were replaced on<br />
day 3. The luteal phase was supported with natural<br />
progesterone (Utrogestan ® ; Besins, France) administered<br />
via the vaginal route (300 mg/day) <strong>com</strong>bined or not with<br />
HCG (1500 IU the day after transfer and 3 days later)<br />
depending on the oestradiol concentration the day of HCG<br />
administration. In total, 291 patients were treated with GH<br />
during the period, of whom 245 were considered for the<br />
<strong>com</strong>parison study. The other 46 patients were excluded<br />
because the stimulation protocol involved either urinary<br />
FSH (n = 15) or clomiphene citrate (n = 2), associations of<br />
rFSH and HMG (n = 21), no gonadotrophin (n = 2), or was<br />
not clearly specified (n = 6).<br />
Comparison group<br />
The patients treated with GH were <strong>com</strong>pared with all<br />
the patients with three or more previous assisted cycle<br />
failures, treated during the same period, without GH, with<br />
a stimulation protocol involving either rFSH only or HMG<br />
only (n = 2780).<br />
The efficacy of GH with rFSH and HMG was analysed.<br />
The total dose of rFSH (IU) or HMG, plasma oestradiol on<br />
the day of HCG, total number and quality of retrieved<br />
cumulus–oocyte–<strong>com</strong>plexes, oocyte quality, particularly<br />
before ICSI (if any) (dark cytoplasm and large blurred<br />
perivitelline space and/or thick zona pellucida), number of<br />
embryos obtained and their quality, number of embryos<br />
replaced, clinical pregnancy rate, number of multiple<br />
pregnancies, miscarriages and live birth rates were<br />
recorded.<br />
Statistical analysis<br />
The analysis was performed in two steps. In the first<br />
step, the two groups were <strong>com</strong>pared with<br />
monovariate analysis, using chi-squared analysis or<br />
variance analysis according to the nature of the variable. In<br />
the second step, a multivariate logistic model was applied<br />
to analyse the effect of the main prognostic factors on the<br />
pregnancy chance per oocyte retrieval and per transfer. In<br />
this case, OR and 95% CI were calculated. Analysis was<br />
carried out using SAS software, release 9.<br />
Results<br />
Patient characteristics<br />
The patients in the GH group were slightly younger<br />
than in the control group and although the difference was<br />
not large it was statistically significant (35.5 years ± 4.0<br />
versus 36.6 ± 4.1, P < 0.001). They also had a significantly<br />
lower FSH dosage on day 3 (6.7 ± 2.0 IU/l, versus 7.1 ± 2.0<br />
IU/l, P = 0.04). The other parameters evaluating the ovarian<br />
reserve were not statistically different in the two groups<br />
(Table 1). The study covers a 5-year period from 2002<br />
(when only a few cycles were included) to 2007. The<br />
percentages of cycles with GH were 5.5, 14.7, 6.7, 9.6, 6.5,<br />
for 2003, 2004, 2005, 2006, and 2007, respectively, without<br />
any significant tendency.<br />
Ovarian response<br />
The average total dose of gonadotrophins (IU) used to<br />
perform stimulation was lower in the GH group (2586 ±<br />
<strong>12</strong>37 versus 2886 ± 1300 in the control group), and the<br />
oestradiol concentration on the day of HCG was higher<br />
(2215 ± <strong>12</strong>86 versus 1945 ± 1132, P < 0.01), whereas the<br />
duration of ovarian stimulation was almost the same<br />
(Table 1).<br />
Efficacy results<br />
The numbers of oocytes retrieved and inseminated<br />
were higher in the GH group (respectively, 10.6 ± 5.9<br />
versus 9.0 ± 5.8, and 8.8 ± 5.5 versus 7.6 ± 5.1, respectively;<br />
P < 0.001 for both), as was the number of embryos cleaved<br />
24 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
at day 2 (5.4 ± 4.0 versus 4.7 ± 3.7, P < 0.001). The cleavage<br />
rates were equivalent, as were the mean numbers of<br />
transferred embryos, while clinical pregnancy rate was<br />
significantly higher in the GH group (25.7% versus 18.7%,<br />
P < 0.01). The effects of GH on enhancing the number of<br />
oocytes retrieved and pregnancy rate were significant<br />
when adjusting for the women’s age (P = 0.01 and P = 0.03,<br />
respectively; Table 2), even if there were few significant<br />
differences in each strata, mainly because of low numbers.<br />
Pregnancy evolution was <strong>com</strong>parable in the two groups,<br />
with 70.4% delivery per pregnancy in the GH group,<br />
<strong>com</strong>pared with 73.2% in the control group. Abortion and<br />
ectopic pregnancy rates were 23.7% versus 25.4% and 1.8%<br />
versus 2.5%, respectively.<br />
Oocyte characteristics<br />
The percentage of atretic oocytes (small, brownish<br />
without visible organelles) was the same in the two groups<br />
(7.5 ± 10.2% in the GH group versus 8.9 ± 14.3% in the<br />
control group), as was the percentage of metaphase II<br />
(MII) oocytes among the retrieved oocytes (79.9 ± 18.4%<br />
versus 81.0 ± 19.6%, respectively). The number of<br />
dysmorphic oocytes was higher in the GH group (3.7 ± 4.6<br />
versus 3.0 ± 3.7, P < 0.01), as was the total number of<br />
oocytes, but the proportion among the MII oocytes was<br />
similar (46.0 ± 42.3% in the GH group versus 46.0 ± 44.5%).<br />
The impact of the gonadotrophin choice (rFSH or<br />
HMG) in association with GH is shown in Table 3. The<br />
numbers of oocytes retrieved and inseminated were<br />
higher in the group GH + rFSH (11.4 ± 6.3 versus 8.8 ± 4.3,<br />
P < 0.01 and 9.5 ± 5.9 versus 7.0 ± 3.7, P < 0.001,<br />
respectively) while the pregnancy rate was the same<br />
(25.4% versus 26.4%) The two groups did not differ<br />
significantly in age or in the ovarian reserve tests.<br />
The multivariate model, applied to take into account<br />
the effect of women’s age and the gonadotrophin nature<br />
on the chances of pregnancy (Table 4) revealed a very<br />
significant effect of age, with a decreased OR of 0.67 (95%<br />
CI 0.53–0.85) for women aged 38–39 years <strong>com</strong>pared with<br />
age 37 years and less, and OR of 0.50 (95% CI 0.40–0.52) for<br />
those aged 40 years or more. The positive effect of GH was<br />
also significant (OR = 1.48, 95% CI 1.<strong>12</strong>–1.96), whereas the<br />
gonadotrophin choice had no significant effect on the<br />
pregnancy chance. A second model including the year of<br />
aspiration did not find any significant effect for year of<br />
aspiration (OR 0.97, 95% CI 0.91–1.03), the odds ratios for<br />
GH, women’s age and gonadotrophins remained at the<br />
same level.<br />
Discussion<br />
This study shows results in favour of using GH in<br />
addition to gonadotrophins in recurrent assisted cycle<br />
failures. In the GH group, the number of oocytes retrieved<br />
and the pregnancy rate were close to those usually<br />
observed in a first cycle and were much higher than in the<br />
<strong>com</strong>parative group with three previous failures, having no<br />
GH co-treatment. It has to be recognized here that this<br />
study has some methodological limitations. The most<br />
important one lies in the protocol itself, since it was not a<br />
double-blinded randomized study. The <strong>com</strong>parative<br />
group was not chosen at random, because of the results of<br />
the preliminary study conducted in the study centre<br />
during the early 2000s, demonstrating that GH addition<br />
enhanced embryonic development in patients with<br />
dysmorphic oocytes and also at least three IVF failures<br />
(Hazout et al., 2003). The patients, knowing those results<br />
before filling and signing the consent form, did not want<br />
to face the probability of being in the placebo group. Thus,<br />
it was decided to use the patients who were not offered<br />
GH as a <strong>com</strong>parison group, which is susceptible to some<br />
bias since the reason for having or not GH may be related<br />
to underlying prognostic factors. The multivariate<br />
analysis, taking into account the main factors, including<br />
women’s age, made it possible to partly bypass this aspect.<br />
Moreover, only FSH was significantly higher in the<br />
<strong>com</strong>parison group among the ovarian reserve testing,<br />
together with age, and the introduction of age in the model<br />
probably also controlled for FSH, since the strong<br />
relationship between age and FSH is well known (Toner et<br />
al., 1993). Thus, the difference in age cannot explain the<br />
difference found for GH. A second bias possibility could<br />
be due to the study length. Recruiting a sufficient number<br />
of cycles needed 5 years because the percentage of cycles<br />
with three previous failures or more is relatively low, and<br />
also because only a few physicians of the centre<br />
participated and the patients had to agree to a new<br />
treatment. However, the percentage of cycles with GH<br />
among the study period did not vary significantly, and the<br />
multilogistic model including the year of aspiration did<br />
not find any significant effect for year of aspiration, and<br />
the OR for GH, women’s age and gonadotrophins<br />
remained at the same level. Finally, a prescription bias<br />
cannot be totally excluded, but the positive results of this<br />
study encourage the design of a new, randomized study,<br />
double-blinded if possible, in order to get the scientific<br />
proof of GH action.<br />
Gonadotrophins are the most important hormones<br />
that regulate major ovarian functions including<br />
folliculogenesis, steroidogenesis and oocyte maturation. In<br />
addition, locally produced growth factors and metabolic<br />
hormones like GH and insulin growth factors play a role<br />
CONTINUED ON PAGE 26<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
25
ARTICLES<br />
CONTINUED FROM PAGE 25<br />
either alone or in <strong>com</strong>bination with gonadotrophins<br />
(Moreira et al., 2002).<br />
The results of this study confirm in humans the<br />
observations obtained by Izadyar on in-vitro maturation<br />
(IVM) of bovine oocytes. These workers found that the<br />
addition of GH in the culture medium accelerates nuclear<br />
maturation and promotes subsequent embryonic<br />
development. GH receptors have been described in mouse<br />
and bovine oocytes and early preimplantation embryos<br />
(Izadyar et al., 1996, 1998, 1999, 2000; Pantaleon et al., 1997).<br />
GH has a positive effect, in animal models, on maturation<br />
process through a favourable increase in cytoplasmic<br />
<strong>com</strong>petence leading to better embryo quality obtained<br />
either in vitro or in vivo (Folch et al., 2001; Moreira et al.,<br />
2002; de Prada and VandeVoort, 2008). The increase of MII<br />
bovine oocytes obtained after 16 h of culture pointed to an<br />
effect of GH on the kinetics of meiosis rather than an<br />
enhancement of the proportion of oocytes that reached to<br />
the MII stage after 24 h of maturation. The acceleration of<br />
meiosis by GH affected the first cleavage divisions rather<br />
than the process of fertilization. mRNA for GH receptor<br />
(GHR) in humans is present in the oocyte and cumulus<br />
cells during the final stage of oocyte maturation (Ménézo<br />
et al., 2003). This argues in favour of a role for GH during<br />
oocyte maturation, especially in terms of cytoplasmic<br />
<strong>com</strong>petence. GHR mRNA is also present in human<br />
embryos during preimplantation development. In<br />
26 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
humans, the results are more controversial: its positive<br />
action seems to be limited to a special population of age<br />
and/or poor-responder patients (Harper et al., 2003;<br />
Tesarik et al., 2005). More recently, it was proposed that<br />
GH supplementation might improve embryo quality in<br />
patients with GH deficiency (Rajesh et al., 2007), fitting<br />
with the previous observations of Mendoza et al. (2002). In<br />
any case, GH increases the maturation rates of human<br />
naked germinal vesicle (GV) oocytes (Hassan et al., 2001;<br />
Ménézo et al., 2003, 2006). It resulted in live births after<br />
maturation of naked GV oocytes (Ménézo et al., 2006) and<br />
it increases the rate of maturation in an IVM programme<br />
(Ali et al., 2006). Mendoza et al. (2002) noted that GH<br />
concentration in follicular fluid is associated with assisted<br />
reproduction treatment failures: it is obvious that, in some<br />
patients, the GH concentration can be below a critical<br />
threshold impairing ovarian function (Spiliotis, 2003),<br />
especially during the late phases of oocyte maturation.<br />
Exogenous GH tends to correct this negative effect (Rajesh<br />
et al., 2007) and the present study suggested that GH may<br />
be effective in assisted reproduction treatment in a specific<br />
group of young women who failed to conceive after at<br />
least three IVF/ICSI cycles without a rationale explanation<br />
and no specific aetiology.<br />
For all patients in the GH group, more than three<br />
transfers of three or more embryos were unsuccessful.<br />
Ovarian response to the <strong>com</strong>bined treatment (GnRHa +<br />
rFSH +rHGH) showed that follicular maturation occurred<br />
at the same time as in cycles without rHGH treatment.<br />
This is not in agreement with previous studies that found<br />
CONTINUED ON PAGE 28<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
27
ARTICLES<br />
CONTINUED FROM PAGE 27<br />
that HGH enhances FSH-induced oestradiol production<br />
by isolated human ovary in vivo (Lanzone et al., 1996),<br />
FSH-independent oestradiol production by isolated<br />
human granulosa cells (Mason et al., 1990) and FSHinduced<br />
formation of LH receptor in rat granulosa cells<br />
(Jia et al., 1986). The total number of good-quality oocytes<br />
and embryos obtained was significantly improved without<br />
a real change in the classical scheme of maturation<br />
(stimulation length, hormone dose, oestradiol<br />
concentration). GH seems to act per se. In this way, this<br />
study seems to be partly in agreement both with the<br />
Cochrane database (Kotarba et al., 2000) rather than the<br />
European–Australian multicentre study on the absence of<br />
effect on stimulation parameters. The increase in the<br />
number of good-quality oocytes collected in the GH group<br />
was not found in the Cochrane analysis. The costimulation<br />
treatment rFSH was as efficient as HMG. The<br />
impact also seems to be related to oocyte quality leading to<br />
better embryo <strong>com</strong>petence in terms of cleavage and<br />
further development to term. GH supplementation in this<br />
group of patients resulted in a pregnancy rate similar to<br />
the overall patient population.<br />
Growth hormone signalling is multiple: it uses the<br />
STAT (Ras/Raf/MEK/extracellular signal-regulated) kinase<br />
pathway, the MAP kinase/CREB pathways. It is interesting<br />
to note that the RAS/RAF/MEK extracellular signalregulated<br />
kinase pathway interacts with the LIF Janus<br />
kinase/STAT pathway: LIF receptor is present in the oocyte<br />
and the preimplantation embryo. GH and LIF increase<br />
gene regulation in cumulus cells and oocytes. A possible<br />
target for this signalling could be the oocyte DNA repair<br />
capacity, which is of major importance, especially for ICSI<br />
for poor sperm quality (Ménézo et al., 2007). Liver and<br />
embryo have many <strong>com</strong>mon metabolic similarities and<br />
GH increases liver DNA repair capacity (Thompson et al.,<br />
2000; Herubel et al., 2002). However a direct effect on the<br />
endometrium positively affecting implantation cannot be<br />
totally excluded. This leads to the idea that HGH plays a<br />
direct role in the oocyte at the final stage of maturation,<br />
particularly in terms of cytoplasmic maturation. In a<br />
recent case report, Ménézo et al. (2006) demonstrated that<br />
pregnancy and delivery could be obtained after IVM of<br />
naked GV human oocytes with HGH and transfer of a<br />
frozen–thawed blastocyst.<br />
In conclusion, although this study was not a<br />
randomized controlled trial, the data obtained here are<br />
indicate a positive effect of GH on IVF out<strong>com</strong>e in a special<br />
population of patients with no clear explanation for their<br />
multiple failure of embryo transfer. These results need to<br />
be confirmed by a large-scale randomized controlled trial.<br />
References<br />
Ali A, Benkhalifa M, Miron P 2006 In-vitro maturation of<br />
oocytes: biological aspects. Reproductive BioMedicine Online<br />
13, 437–446.<br />
de Matos DG, Miller K, Scott R et al. 2008 Leukemia inhibitory<br />
factor induces cumulus expansion in immature human and<br />
mouse oocytes and improves mouse two-cell rate and<br />
delivery rates when it is present during mouse in vitro<br />
oocyte maturation. Fertility and Sterility 90, 2367–2375.<br />
de Prada JK, VandeVoort CA 2008 Growth hormone and in-vitro<br />
maturation of rhesus macaque oocytes and subsequent<br />
embryo development. Journal of Assisted Reproduction and<br />
Genetics 25, 145–158.<br />
Folch J, Ramón JP, Cocero MJ et al. 2001 Exogenous growth<br />
hormone improves the number of transferable embryos in<br />
superovulated ewes. Theriogenology 55, 1777–1785.<br />
Harper K, Proctor M, Hughes E 2003 Growth hormone for invitro<br />
fertilization. Cochrane Database of Systematic Reviews<br />
CD000099.<br />
Hassan HA, Azab H, Rahman AA, Nafee TM 2001 Effects of<br />
growth hormone on in-vitro maturation of germinal vesicle<br />
human oocytes retrieved from small antral follicles. Journal<br />
of Assisted Reproduction and Genetics 18, 417–420.<br />
Hazout A, Junca AM, Tesarik J, Ménézo JR 2003 Effet promoteur<br />
de l’hormone de croissance sur la <strong>com</strong>pétence des ovocytes<br />
dysmorphiques: résultats d’une étude pilote randomisée en<br />
double aveugle. Références en Gynécologie et Obstétrique 10,<br />
71–77.<br />
Herubel F, El Mouatassim S, Guerin P et al. 2002 Genetic<br />
expression of monocarboxylate transporters during human<br />
and murine oocyte maturation and early embryonic<br />
development. Zygote 10, 175–181.<br />
Howles CM, Loumaye E, Germond M et al. 1999 Does GH<br />
releasing factor assist follicular development in poor<br />
responder patients undergoing ovarian stimulation for IVF<br />
Human Reproduction 9, 1939–1943.<br />
Hsu CJ, Hammond JM 1987 Con<strong>com</strong>itant effects of growth<br />
hormone on secretion of insulin-like growth factor I and<br />
progesterone by cultured porcine granulosa cells.<br />
Endocrinology <strong>12</strong>1, 1343-1348.<br />
Izadyar F, Van Tol HTA, Hage WG, Bevers MM 2000<br />
Preimplantation bovine embryos express mRNA of GH<br />
receptor and respond to GH addition during in-vitro<br />
development. Molecular Reproduction and Development 57,<br />
247–255.<br />
Izadyar F, Zhao J, Van Tol HTA, Colenbrander B, Bevers MM<br />
1999 Messenger mRNA expression and protein localization<br />
of growth hormone in bovine ovarian tissue and in cumulus<br />
oocyte <strong>com</strong>plexes during in-vitro maturation. Molecular<br />
Reproduction and Development 53, 398–407.<br />
Izadyar F, Hage WJ, Colenbrander B, Bevers MM 1998 The<br />
promotory effect of growth hormone on developmental<br />
<strong>com</strong>petence of in-vitro matured bovine oocytes is due to<br />
improved cytoplasmic maturation. Molecular Reproduction<br />
and Development 49, 444–453.<br />
Izadyar F, Colenbrander B, Bevers MM 1996 In-vitro maturation<br />
of bovine oocytes in the presence of growth hormone<br />
accelerates nuclear maturation and promotes subsequent<br />
28 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
embryonic development. Molecular Reproduction and<br />
Development 45, 372–377.<br />
Jia X, Kalmingin J, Hsueh AJW 1986 Growth hormone enhances<br />
follicle stimulating hormone-induced differentiation of<br />
cultured rat granulosa cells. Endocrinology 118, 1401–1409.<br />
Kotarba D, Kotarba J, Hughes E 2000 Growth hormone for in<br />
vitro fertilization. Cochrane Database of Systematic Reviews,<br />
CD000099.<br />
Langhout DJ, Spicer LJ, Geisert RD 1991 Development of a<br />
culture system of granulosa cells: effects of growth hormone<br />
estradiol and gonadotropins on cell proliferation<br />
steroidogenesis and protein synthesis. Journal of Animal<br />
Science 69, 3321–3334.<br />
Lanzone A, Fortini A, Fulghesu AM et al. 1996 Growth hormone<br />
enhances estradiol production follicle-stimulating hormone<br />
induced in the early stage of follicular maturation. Fertility<br />
and Sterility 66, 948–953.<br />
Mason HD, Martikainen H, Beard RW et al. 1990 Direct<br />
gonadotropin effect of growth hormone on estradiol<br />
production by human granulosa cells. Journal of<br />
Endocrinology <strong>12</strong>6, R1–R4.<br />
Mendoza C, Ruiz Requena E et al. 2002 Follicular fluid markers<br />
of oocyte developmental potential. Human Reproduction 17,<br />
1017–1022.<br />
Ménézo YJ, Russo R, Tosti G et al. 2007 Expression profile of<br />
genes coding for DNA repair in human oocytes using<br />
pangenomic microarrays, with a special focus on ROS linked<br />
decays. Journal of Assisted Reproduction and Genetics 24,<br />
513–520.<br />
Ménézo YJ, Nicollet B, Rollet J, Hazout A 2006 Pregnancy and<br />
delivery after in-vitro maturation of naked ICSI-GV oocytes<br />
with GH and transfer of a frozen thawed blastocyst: case<br />
report. Journal of Assisted Reproduction and Genetics 23, 47–49.<br />
Ménézo YJ, el Mouatassim S, Chavrier M et al. 2003 Human<br />
oocytes and preimplantation embryos express mRNA for<br />
growth hormone receptor. Zygote 11, 293–297.<br />
Modina S, Borromeo V, Luciano AM et al. 2007 Relationship<br />
between growth hormone concentrations in bovine oocytes<br />
and follicular fluid and oocyte developmental <strong>com</strong>petence.<br />
European Journal of Histochemistry 51, 173–180.<br />
Moreira F, Paula-Lopes FF, Hansen PJ et al. 2002 Effects of growth<br />
hormone and insulin like factor-1 on development of invitro<br />
derived bovine embryos. Theriogenology 57, 895–907.<br />
Pantaleon M, Whiteside EJ, Harvey MB et al. 1997 functional GH<br />
receptors and GH are expressed by preimplantation mouse:<br />
a role for GH in early embryogenesis Proceedings of the<br />
National Academy of Science of the United States of America 94,<br />
5<strong>12</strong>5–5130.<br />
Ptak G, Lopes F, Matsukawa K et al. 2006 Leukaemia inhibitory<br />
factor enhances sheep fertilization in vitro via an influence<br />
on the oocyte. Theriogenology 65, 1891–1899.<br />
Rajesh H, Yong YY, Zhu M et al. 2007 Growth hormone deficiency<br />
and supplementation at in-vitro fertilisation. Singapore<br />
Medical Journal 48, 514–518.<br />
Schoolcraft W, Schlenker T, Gee M 1997 improved controlled<br />
ovarian hyperstimulation in poor responders IVF patients<br />
with a microdose follicle stimulating hormone flare, growth<br />
hormone protocol. Fertility and Sterility 67, 93–97.<br />
Spiliotis BE 2003 Growth hormone insufficiency and its impact<br />
on ovarian function. Annals of the New York Academy of<br />
Sciences 997, 77–84.<br />
Tesarik J, Hazout A, Mendoza C 2005 Improvement of delivery<br />
and live birth rates after ICSI in women aged >40 years by<br />
ovarian co-stimulation with growth hormone. Human<br />
Reproduction 20, 2536–2541.<br />
Ptak G, Lopes F, Matsukawa K et al. 2006 Leukaemia inhibitory<br />
factor enhances sheep fertilization in vitro via an influence<br />
on the oocyte. Theriogenology 65, 1891–1899.<br />
Rajesh H, Yong YY, Zhu M et al. 2007 Growth hormone deficiency<br />
and supplementation at in-vitro fertilisation. Singapore<br />
Medical Journal 48, 514–518.<br />
Schoolcraft W, Schlenker T, Gee M 1997 improved controlled<br />
ovarian hyperstimulation in poor responders IVF patients<br />
with a microdose follicle stimulating hormone flare, growth<br />
hormone protocol. Fertility and Sterility 67, 93–97.<br />
Spiliotis BE 2003 Growth hormone insufficiency and its impact<br />
on ovarian function. Annals of the New York Academy of<br />
Sciences 997, 77–84.<br />
Tesarik J, Hazout A, Mendoza C 2005 Improvement of delivery<br />
and live birth rates after ICSI in women aged >40 years by<br />
ovarian co-stimulation with growth hormone. Human<br />
Reproduction 20, 2536–2541.<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
29
FDA 510(k) Cleared<br />
INTRODUCING THE<br />
global ® DMSO Blastocyst Vitrification System<br />
global ® DMSO Blastocyst Vitrification Kit<br />
• 2 X 1 ml vial of Equilibration Solution containing DMSO, Ethylene Glycol and 10 mg/ml HSA in global ® w/HEPES<br />
• 2 X 1 ml vial of Vitrification Solution containing DMSO, Ethylene Glycol, Sucrose, and 10 mg/ml HSA in global ® w/HEPES<br />
• 4 X SunIVF embyo GPS ® dishes, sterile, endotoxin and MEA tested<br />
global ® DMSO Blastocyst Warming Kit<br />
• 1 X 1 ml vial of Warm 1 Solution containing Sucrose and 10 mg/ml HSA in global ® w/HEPES<br />
• 1 X 1 ml vial of Warm 2 Solution containing Sucrose and 10 mg/ml HSA in global ® w/HEPES<br />
• 2 X 1 ml vial of Warm 3 Solution containing 10 mg/ml HSA in global ® w/HEPES<br />
• 4 X SunIVF embryo GPS ® dishes, sterile, endotoxin and MEA tested<br />
Reference<br />
Stehlik E, Stehlik J, Katayama KP, Kuwayama M, Jambor V, Brohammer R, Kato O (2005) Vitrification demonstrates significant<br />
improvement versus slow freezing of human blastocysts. Reprod Biomed Online 11, 53-7.<br />
US/Canada: 1-800-720-6375 • International: 1-519-826-5800 •Fax: 1-519-826-6947<br />
email: sales@LifeGlobal.<strong>com</strong> • www.LifeGlobal.<strong>com</strong>
INTRODUCING THE<br />
global ® Blastocyst Vitrification System – Based on S 3<br />
global ® Blastocyst Vitrification Kit – Based on S 3<br />
• 2 X 1 ml vial of Vitrification Solution 1 • 5 X 1.2 ml vial of Vitrification Solution 3<br />
• 2 X 1 ml vial of Vitrification Solution 2 • 4 X SunIVF Universal GPS ® dishes<br />
global ® Blastocyst Vitrification Thawing Kit – Based on S 3<br />
• 1 X 1 ml vial of Thawing Solution 1 • 1 X 1 ml vial of Thawing Solution 4<br />
• 1 X 1 ml vial of Thawing Solution 2 • 1 X 1 ml vial of Thawing Solution 5<br />
• 1 X 1 ml vial of Thawing Solution 3 • 4 X SunIVF Universal GPS ® dishes<br />
References<br />
Stachecki JJ, Cohen J (2008) S 3 Vitrifcation System: A novel approach to blastocyst freezing. J. Clin. Embryol. 11, 5-14.<br />
Stachecki JJ, Garrisi J, Sabino S et al. 2008 A new safe, simple and successful vitrification method for bovine and human<br />
blastocysts. Reprod Biomed Online 17, 360-367.<br />
US/Canada: 1-800-720-6375 • International: 1-519-826-5800 •Fax: 1-519-826-6947<br />
email: sales@LifeGlobal.<strong>com</strong> • www.LifeGlobal.<strong>com</strong>
ARTICLES<br />
S 3 Vitrification System: a Novel Approach to<br />
Blastocyst Freezing<br />
James J. Stachecki, Ph.D., Jacques Cohen, Ph.D.<br />
Tyho-Galileo Research Laboratories, 3 Regent Street, Suite 301, Livingston, NJ 07039<br />
Email: james @galileoivf.<strong>com</strong><br />
Stachecki JJ, Cohen J (2008) S 3 vitrification system: A novel approach to blastocyst freezing. J. Clin. Embryol, 11, 5-14<br />
This article was first published in The Journal of Clinical Embryology, 11, 5-14 (2008). It is reproduced here with permission from The<br />
Journal of Clinical Embryology.<br />
Reducing multiple pregnancies is a concern of IVF<br />
clinics everywhere. Blastocyst transfer is the<br />
method of choice for the replacement of just a<br />
single embryo. As more clinics be<strong>com</strong>e proficient at<br />
culturing embryos to the blastocyst stage there is an<br />
increasing need to store extra blastocysts. Slow-cooling<br />
regimes have been around for over 30 years, and<br />
although thousands of babies have been produced<br />
throughout the world from this technique, there is room<br />
for improvement, especially when it <strong>com</strong>es to storing<br />
blastocysts. Modern, faster methods of cryopreservation,<br />
namely vitrification, are enticing because of their<br />
apparent ease, reduced procedure time, and published<br />
success rates. Rapid freezing has been the focus of<br />
research in recent years and, in several laboratories, is<br />
now the preferred method for storing human embryos.<br />
The concept of vitrification, or achieving a glasslike<br />
state, is not new and was first described in 1860. Rall and<br />
Fahy showed that vitrification is a potential alternative to<br />
slow-cooling of embryos over 100 years later (Rall and<br />
Fahy, 1985). Since then it has been the topic of numerous<br />
publications in the IVF field (Ali, 2001; Antinori et al.,<br />
2007; Chen et al., 2000; Chung et al., 2000; Cremades et al.,<br />
2004; Hiraoka et al., 2004; Kasai and Mukaida, 2004;<br />
Kuleshova and Lopata, 2002; Liebermann and Tucker,<br />
2002; Liebermann et al., 2003; Vanderzwalmen et al., 2003;<br />
Vanderzwalmen et al., 2002; Wininger and Kort, 2002; Wu<br />
et al., 2001; Yoon et al., 2003). Vitrification by rapid<br />
cooling proved the only effective method to store the<br />
cold-sensitive oocytes and embryos of pigs, cows, and<br />
sheep (Beeb et al., 2002; Fahning and Garcia, 1992; Szell et<br />
al., 1990; Vajta et al., 1998). Soon after these reports were<br />
published, the method of vitrification by rapid cooling<br />
was applied to human embryo storage. Many of the<br />
recent manuscripts clearly show improved results in<br />
terms of survival and clinical pregnancy rates, when<br />
using vitrification. However, current vitrification<br />
methods have potential problems.<br />
In order to understand rapid-cooling or vitrification<br />
techniques, let us <strong>com</strong>pare them to the slow-cooling<br />
method. During slow-cooling using a programmable<br />
freezer, embryos are exposed to relatively low<br />
concentrations of permeable and non-permeable<br />
cryoprotectants (such as 1.5M Propanediol (PrOH) in<br />
conjunction with 0.2M sucrose), equilibrated for 10-25 min<br />
at room temperature, loaded into a straw or vial, sealed<br />
and placed into a controlled-rate freezer. Ice formation is<br />
initially induced extracellularly by seeding and, as a result<br />
of the solute gradient created, freezable water flows out of<br />
the cells minimizing the chance of intracellular ice<br />
formation during cooling. As the temperature is gradually<br />
lowered, the concentration of cryoprotectant in the liquid<br />
phase (which includes intracellular fluid) increases<br />
correspondingly until a level is reached at which<br />
additional formation and growth of ice crystals, although<br />
possible, are unlikely even if the temperature drops<br />
further (Luyet, 1970). Rather, this remaining liquid phase<br />
turns immediately into a glassy substance upon plunging<br />
into liquid nitrogen and solidifies without further crystal<br />
formation. The unfrozen liquid phase remaining within<br />
the cells should now ideally consist of this glassy<br />
substance with all original cell solutes remaining in<br />
solution (Luyet, 1970). This suggests that when we slowcool<br />
cells using a penetrating cryoprotectant such as<br />
PrOH, and standard slow-cooling protocols, we are<br />
actually vitrifying the cells. Indeed, when we slow-cool<br />
human embryos, typical survival rates range between 80%<br />
and 100%, for many IVF centers. These survival rates<br />
would not be possible, at least according to Mazur<br />
(Mazur, 1963), if intracellular ice formation were<br />
occurring. This correlates well with the theory that<br />
slowcooling is vitrification. Now let’s examine modern<br />
day rapid-cooling (vitrification). All of the rapid-cooling<br />
forms of vitrification procedures for human embryos<br />
described in the recent literature are, in principle, the<br />
same. They all involve exposure of oocytes or embryos to<br />
high concentrations of cryoprotectant(s) for brief periods<br />
of time at or near room temperature followed by loading<br />
onto or into a tiny container (cryo-loop, cryo-top, cryoleaf,<br />
cryo-tip, etc.) that may or may not be sealed, then<br />
submerged directly into liquid nitrogen and stored. The<br />
high osmolarity of the vitrification solution rapidly<br />
32 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
dehydrates the cell, some of the cryoprotectant enters the<br />
cell and binds the remaining water, and submersion into<br />
liquid nitrogen quickly solidifies or vitrifies the cell so that<br />
any remaining intracellular water not bound by<br />
cryoprotectants, does not have time to form a lethal<br />
amount of ice crystals. The embryo is effectively vitrified<br />
without intracellular ice, similar to slow-cooling. From<br />
these descriptions, both techniques, although seemingly<br />
very different, have the same out<strong>com</strong>e of vitrifying the cell.<br />
Therefore, the term vitrification can be used to describe<br />
both slow-cooling or rapid-cooling, as long as the out<strong>com</strong>e<br />
is the formation of an amorphous glass-like solid.<br />
Compared with slow-cooling, rapid cooling<br />
vitrification has allowed for improved survival and<br />
pregnancy rates. Reasons for this are numerous, despite<br />
the fact that both procedures vitrify the cell. The methods<br />
are different enough that, despite posing a greater risk<br />
from the potential toxicity of the highly concentrated<br />
cryoprotectants used and the relatively high exposure<br />
temperature, rapid-cooling has met with greater success in<br />
most instances (Hotamisligil et al., 1996; Mukaida et al.,<br />
1998). To <strong>com</strong>bat the toxic effects of elevated<br />
cryoprotectant levels, exposure to the final vitrification<br />
solution is usually limited to around 45-90 seconds or less<br />
before plunging in liquid nitrogen (Chung et al., 2000;<br />
Hong et al., 1999; Hunter et al., 1995; Shaw et al., 1992; Wu<br />
et al., 2001; Yoon et al., 2003). Also, to promote faster<br />
solidification, minute amounts of vitrification media are<br />
used, usually under 2ul. For example, when using the<br />
cryo-top device, the cell(s) are placed on the tip and excess<br />
media is removed leaving the cell(s) covered in a very thin<br />
film of media before plunging directly into liquid nitrogen<br />
This allows for an extremely rapid cooling rate of over<br />
20,000°C/min as shown in Table 1.<br />
Similar vitrification devices to those in Table 1 allow<br />
cooling rates of >15,000°C/min and have resulted in high<br />
survival rates (Antinori et al., 2007; Cremades et al., 2004;<br />
Hiraoka et al., 2004; Hong et al., 1999; Huang et al., 2005;<br />
Isachenko et al., 2005; Kuwayama et al., 2005a; Lane and<br />
Gardner, 2001; Liebermann et al., 2003; Martino et al., 1996;<br />
Mukaida et al., 2003; Son et al., 2003; Wu et al., 2001). In<br />
fact, the <strong>com</strong>bination of cryoprotectants used in<br />
conjunction with very rapid cooling rates has allowed for<br />
these results (Table 2), whereas slower cooling rates have<br />
yielded poor survival rates (Escriba et al., 2006;<br />
Vanderzwalmen et al., 2002).<br />
Despite the increase in survival and pregnancy rates,<br />
and relative abundance of recent reports on rapid-cooling<br />
vitrification (Table 2), there are numerous potential<br />
short<strong>com</strong>ings associated with these protocols that have<br />
prevented its widespread application and acceptance<br />
(Kuleshova and Lopata, 2002). Viral contamination from<br />
direct contact to liquid nitrogen is a concern despite<br />
Table 1. Cooling rates for modern vitrification devices.<br />
Device Media (ul) Freezing Rate<br />
0.25cc straw 25ul 4460°C/min<br />
Open-pulled straw 1.5ul 16,340°C/min<br />
Cryo-Top 0.1ul 22,800°C/min<br />
Cryo-Tip
ARTICLES<br />
CONTINUED FROM PAGE 33<br />
Table 2. Published survival and pregnancy rates of vitrified human cells.<br />
Study Cell Type n Survival Rate (%) Pregnancy Rate (%)<br />
(Lane et al., 1999) Blastocyst 18 83% N/A<br />
(Choi et al., 2000) Blastocyst 93 51.6% 25%<br />
(Cho et al., 2002) Blastocyst <strong>12</strong>0 84.2% 34.1%<br />
(Reed et al., 2002) Blastocyst 15 100% 25%<br />
(Vanderzwalmen et al., 2002) Blastocyst 75 70.6% 22.9%<br />
(Vanderzwalmen et al., 2003) Blastocyst 186 78.5% 32.4%<br />
(Mukaida et al., 2003) Blastocyst 725 80.4% 37%<br />
(Son et al., 2003) Blastocyst 90 90 48%<br />
(Cremades et al., 2004) Blastocyst 33 82% N/A<br />
Teramoto et al., 2004 (abs) Blastocyst 197 100 57.7%<br />
(Hiraoka et al., 2004) Blastocyst 49 98% 50%<br />
(Huang et al., 2005) Blastocyst 96 77.1% 53.8%<br />
(Takahashi et al., 2005) Blastocyst 1<strong>12</strong>9 85.7% 44.1%<br />
(Stehlik et al., 2005) Blastocyst 41 100 50%<br />
(Kuwayama et al., 2005a) Blastocyst 6328 90 53%<br />
(Liebermann and Tucker, 2006) Blastocyst 547 96.5% 46.1%<br />
(Stachecki et al., 2008) Blastocyst 93 89 65%<br />
(Isachenko et al., 2003) Embryo 59 71 N/A<br />
(Kuwayama et al., 2005a) Embryo 5881 100% 44%<br />
(Kuwayama et al., 2005a) Embryo 897 98 32<br />
(Sher et al., 2008) Embryo 78 96% 63%<br />
(Balaban et al., 2008) Embryo 234 94.8% 49%<br />
(Katayama et al., 2003) Oocyte 46 94% 33.3%<br />
(Kuwayama et al., 2005b) Oocyte 64 91% 45.4%<br />
(Selman et al., 2006) Oocyte 24 75% 33%<br />
(Lucena et al., 2006) Oocyte 159 96.7% 56.5%<br />
(Antinori et al., 2007) Oocyte 330 99% 32.5%<br />
(Cobo et al., 2008) Oocyte 27 86.9% N/A<br />
Cao et al., 2008 Oocyte 292 91.8% N/A<br />
pipeting the blastocyst in and out of a fine bore pipette or<br />
rupturing it using an ICSI needle or similar device<br />
(Hiraoka et al., 2004; Son et al., 2003; Vanderzwalmen et<br />
al., 2002). Although survival can be increased using these<br />
methods, the obvious drawback is that there is an<br />
additional step involved that is potentially damaging to<br />
the embryo.<br />
In this study we describe a new method to vitrify<br />
human blastocysts that is safe, successful, and relatively<br />
easy to learn and use. This method and media can be used<br />
to vitrify blastocysts of all stages (from cavitating to fully<br />
hatched) without reduction of the blastocoel or using<br />
DMSO in the media. Our technique uses a standard 0.25cc<br />
sterile straw, up to 3 times longer cryoprotectant exposure,<br />
ample loading time, and heat-sealing protection. These<br />
factors should permit adequate recovery time in cases of<br />
operator-error. This alternative method to slow-cooling,<br />
entitled S 3 -vitrification, has been described in a recent<br />
study of ours (Stachecki et al., 2008) and we report here<br />
updated clinical out<strong>com</strong>es from several clinics using this<br />
technique.<br />
Materials and Methods<br />
Collection of Blastocysts<br />
Luteal phase GnRH agonist regimes, also called luteal<br />
phase lupron protocols, were used for all patient hormonal<br />
stimulations and oocytes were collected by standard<br />
means with fertilization occurring using ICSI or IVF. Only<br />
high quality blastocysts that had a well formed blastocoel,<br />
trophectoderm with many cells, and a well-formed visible<br />
ICM were chosen for clinical vitrification. The Gardner<br />
scale was used to grade blastocysts, where the ICM or<br />
34 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
trophectoderm was either an A or B (Gardner et al., 2000).<br />
None of the patients had HCV.<br />
Training<br />
Several embryologists at 5 different IVF clinics in<br />
North and South America were trained in the S3<br />
Vitrification procedure over a period of 1 to 2 days. After<br />
training, the embryologists practiced vitrifying spare<br />
blastocysts consented for research. Upon reaching a level<br />
of <strong>com</strong>fort with the technique and their results, which<br />
varied in time from 1 week to 2 months (depending on<br />
how much material was available to practice with) they<br />
began clinical use.<br />
Vitrification<br />
A series of 3 solutions (V1, V2, V3) were used to vitrify<br />
blastocysts according to (Stachecki et al., 2008). Blastocysts<br />
were exposed to V1 for 5 min at room temperature (RT),<br />
transferred to V2 for 5 min at RT, and then to V3. Once in<br />
V3, the cells were immediately loaded into a standard,<br />
sterile 0.25cc cryopreservation straw Figure 1. The straws<br />
were frozen per straw.<br />
Thawing and Embryo Replacement<br />
Straws were thawed by holding them in room<br />
temperature air before immersion in a water bath<br />
(Stachecki et al., 2008). After thawing, the cryoprotectants<br />
were removed by dilution at room temperature through a<br />
series of five media (T1-T5) at 5 min per step. The<br />
blastocysts were warmed on a heated surface (37°C)<br />
before being placed in culture at 37°C and then analyzed<br />
before being transferred. Embryos deemed to have<br />
survived thawing were selected for replacement on an<br />
individual patient basis, per the clinic’s guidelines.<br />
Results<br />
Table 3 shows each clinic’s initial results with S3<br />
vitrification along with the totals for all five clinics<br />
<strong>com</strong>bined. Note that these results are from the first set of<br />
blastocysts that the clinics had vitrified and extensive<br />
training and practice was not necessary. Additionally,<br />
there were multiple embryologists performing the<br />
Figure 1. Straw Loading Diagram<br />
were then heat-sealed at both ends. A 0.5cc straw with<br />
patient information was heat-sealed to one end of the<br />
0.25cc straw. The total time it took to load a straw and seal<br />
it was under <strong>12</strong>0 seconds. Straws were then vitrified by<br />
pre-cooling in liquid nitrogen vapors (–95°C to –105°C)<br />
before being stored in liquid nitrogen. This method of<br />
loading and cooling was simple and easily ac<strong>com</strong>plished,<br />
within the given time frame, and in most cases there was<br />
time to spare prior to cooling. Either 1 or 2 blastocysts<br />
vitrification and thaw procedures at each clinic. A total of<br />
884 blastocysts were thawed. Overall survival rates<br />
ranged between 83% and 100% with the average being<br />
88.7%. Fetal heart beat (FHB) rates ranged between 32.8%<br />
and 58.7%. Pregnancy rates per transfer ranged between<br />
CONTINUED ON PAGE 36<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
35
ARTICLES<br />
CONTINUED FROM PAGE 35<br />
Table 3. Clinical results of S3 Vitrification on blastocysts.<br />
Clinic Thawed Intact Transfers Replaced FHB Preg/Transfer<br />
A 104 86 (83%) 45 80 47 (58.7%) 32/45 (71.1%)<br />
B 160 141 (88.1%) 77 131 43 (32.8%) 37/77 (48.0%)<br />
C 41 35 (85.4%) 19 35 16 (45.7%) <strong>12</strong>/19 (63.1%)<br />
D 566 509 (89.9%) 209 509 N/A 116/209 (55.5%)<br />
E 13 13 (100%) 8 13 5 (38.5%) 5/8 (62.5%)<br />
Total 884 784 (88.7%) 358 768 111 (42.8%) 202/358 (56.4%)<br />
48-71% with the average being 56.4%. All babies born to<br />
date were born healthy with no reported abnormalities.<br />
Discussion<br />
Previous reports show that fast-rate vitrification of<br />
blastocysts offers a feasible and often better approach to<br />
storage than slow-cooling techniques (Table 2).<br />
Refinement of earlier methods has led to the use of tiny<br />
containers and rapid cooling rates that coincide with a<br />
marked increase in blastocyst survival. However, these<br />
procedures are not as easy to use for some individuals and<br />
have other potential problems as described above.<br />
Concerns regarding sterility and ease of use will continue<br />
to grow as more and more regulations are placed upon<br />
IVF clinics by outside inspecting bodies such as CAP and<br />
the FDA. An alternative methodology that avoids these<br />
problems, conforms to potential future regulations, and<br />
provides for high survival and pregnancy rates, would be<br />
very useful. The new technique, S 3 -vitrification, described<br />
here and elsewhere (Stachecki et al., 2008), has been used<br />
effectively and reproducibly, at least based upon the few<br />
clinics that have used this method. The relatively high<br />
survival and pregnancy rates obtained from five clinics in<br />
North and South America were collected from their initial<br />
attempts at using the procedure and, with continued use,<br />
could improve further.<br />
The results varied between clinics, but overall, the<br />
rates were similar to other reports in the scientific<br />
literature. Although the slow-cool vitrification procedure<br />
was similar between the clinics, differences in patient<br />
selection, stimulation, patient age, methods of grading<br />
embryo survival, etc. could all be expected to differ among<br />
embryologists and clinics. For example, Clinic A had the<br />
lowest survival rate with 83%, yet had the highest clinical<br />
pregnancy rate with 71.1%, over a reasonable amount of<br />
transfers (n=45). They appear to have a much more<br />
stringent grading system, and even though survival<br />
seemed to be lower, the blastocysts that they deemed to<br />
have survived led to the highest pregnancy rate among the<br />
clinics. We cannot, and did not want to control for<br />
individual differences within and between clinics. We feel<br />
that this strategy will lead to a more accurate picture of<br />
overall success. Likewise, not every clinic will have the<br />
same success rate for embryo culture, despite the use of<br />
the same culture medium. The data from Table 2 also show<br />
a high variability of survival (51.6% to 100%) and<br />
pregnancy rates (22.9% to 55.6%) for blastocyst storage,<br />
despite the fact that the techniques are essentially the<br />
same. A storage technique that is highly effective and<br />
robust should produce results that, despite the myriad<br />
differences within and between labs, will ultimately<br />
produce reasonably high pregnancy rates. Although there<br />
are higher survival rates reported (see Table 2) collectively,<br />
our results are similar to those reported. Rather than<br />
spending time making extensive <strong>com</strong>parisons to other<br />
studies, the technique of<br />
S 3 vitrification simply represents an alternative method of<br />
storing embryos (and oocytes, data not shown), that can<br />
potentially improve out<strong>com</strong>es and has collectively yielded<br />
over 200 pregnancies to date.<br />
What makes S 3 vitrification unique is that it uses a<br />
relatively large 0.25cc straw, that can be loaded and sealed<br />
easily in a timely manner, and that uses a significantly<br />
slower cooling rate of
ARTICLES<br />
removed and enough cryoprotectant is around to prevent<br />
damage to the trophectoderm and ICM cells based on the<br />
survival and pregnancy rates obtained. After thawing and<br />
removal of cryoprotectants, blastocysts could easily be<br />
graded, and were replaced anytime from 30 minutes to 2<br />
hours after thawing.<br />
The results from the various clinics presented here<br />
demonstrate that blastocysts can be vitrified using a<br />
simple easy-to-use protocol, in a relatively large, sterile,<br />
sealable container without the need for DMSO, and in a<br />
manner that allows time for equilibration and loading. So<br />
far, S 3 vitrification seems to be easy to learn, effective, and<br />
reproducible, yielding high survival and pregnancy rates<br />
in a number of reproductive clinics using the procedure<br />
for the first time.<br />
Acknowledgements<br />
We thank all of the embryologists at the Institute for<br />
Reproductive Medicine and Science, Highland Park IVF<br />
Center, Northwest Center for Reproductive Sciences,<br />
ProCriar, Advanced Reproductive Science, and Bryn<br />
Mawr Center for Reproductive Medicine (Main Line<br />
Health) for their assistance. We also thank all those who<br />
have supported our endeavors and are currently<br />
investigating the use of S 3 vitrification in their clinics.<br />
Many thanks to everyone at Tyho-Galileo Research<br />
Laboratories for their continued support and hard work.<br />
Also, to those women who consented to have their<br />
blastocysts frozen with our technique, we wish you the<br />
best and a healthy and happy pregnancy.<br />
References<br />
Ali J 2001 Vitrification of embryos and oocytes with 5.5 mol/l<br />
ethylene glycol and 1.0 mol/l sucrose. Hum Reprod 16, 1777-<br />
1779.<br />
Antinori M, Licata E, Dani G et al. 2007 Cryotop vitrification of<br />
human oocytes results in high survival rate and healthy<br />
deliveries. Reprod Biomed Online 14, 72-79.<br />
Balaban B, Urman B, Ata B et al. 2008 A randomized controlled<br />
study of human Day 3 embryo cryopreservation by slow<br />
freezing or vitrification: vitrification is associated with<br />
higher survival, metabolism and blastocyst formation. Hum<br />
Reprod 23, 1976-1982. Epub 2008 Jun 1910.<br />
Beeb LF, Cameron RD, Blackshaw AW et al. 2002 Piglets born<br />
from centrifuged and vitrified early and peri-hatching<br />
blastocysts. Theriogenology 57, 2155-2165.<br />
Bielanski A, Bergeron H, Lau PC et al. 2003 Microbial<br />
contamination of embryos and semen during long term<br />
banking in liquid nitrogen. Cryobiology 46, 146-152.<br />
Bielanski A, Nadin-Davis S, Sapp T et al. 2000 Viral<br />
contamination of embryos cryopreserved in liquid nitrogen.<br />
Cryobiology 40, 110-116.<br />
Cao Y, Xing Q 2008 Comparison of survival and embryonic<br />
development in human oocytes cryopreserved by slowfreezing<br />
and vitrification. Fertil Steril 90, S270.<br />
Chen SU, Lien YR, Chao K et al. 2000 Cryopreservation of mature<br />
human oocytes by vitrification with ethylene glycol in<br />
straws. Fertil Steril 74, 804-808.<br />
Cho HJ, Son WY, Yoon SH et al. 2002 An improved protocol for<br />
dilution of cryoprotectants from vitrified human blastocysts.<br />
Hum Reprod 17, 2419-2422.<br />
Choi DH, Chung HM, Lim JM et al. 2000 Pregnancy and delivery<br />
of healthy infants developed from vitrified blastocysts in an<br />
IVF-ET program. Fertil Steril 74, 838-839.<br />
Chung HM, Hong SW, Lim JM et al. 2000 In vitro blastocyst<br />
formation of human oocytes obtained from unstimulated<br />
and stimulated cycles after vitrification at various<br />
maturational stages. Fertil Steril 73, 545-551.<br />
Cobo A, Perez S, De los Santos MJ et al. 2008 Effect of different<br />
cryopreservation protocols on the metaphase II spindle in<br />
human oocytes. Reprod Biomed Online 17, 350-359.<br />
Cremades N, Sousa M, Silva J et al. 2004 Experimental<br />
vitrification of human <strong>com</strong>pacted morulae and early<br />
blastocysts using fine diameter plastic micropipettes. Hum<br />
Reprod 19, 300-305.<br />
Escriba MJ, Escobedo-Lucea C, Mercader A et al. 2006<br />
Ultrastructure of preimplantation genetic diagnosis-derived<br />
human blastocysts grown in a coculture system after<br />
vitrification. Fertility and Sterility 86, 664-671.<br />
Fahning ML, Garcia MA 1992 Status of cryopreservation of<br />
embryos from domestic animals. Cryobiology 29, 1-18.<br />
Hiraoka K, Hiraoka K, Kinutani M et al. 2004 Blastocoele collapse<br />
by micropipetting prior to vitrification gives excellent<br />
survival and pregnancy out<strong>com</strong>es for human day 5 and 6<br />
expanded blastocysts. Hum Reprod 19, 2884-2888. Epub 2004<br />
Sep 2883.<br />
Hong SW, Chung HM, Lim JM et al. 1999 Improved human<br />
oocyte development after vitrification: a <strong>com</strong>parison of<br />
thawing methods. Fertil Steril 72, 142-146.<br />
Hotamisligil S, Toner M, Powers RD 1996 Changes in membrane<br />
integrity, cytoskeletal structure, and developmental<br />
potential of murine oocytes after vitrification in ethylene<br />
glycol. Biology of Reproduction 55, 161-168.<br />
Huang CC, Lee TH, Chen SU et al. 2005 Successful pregnancy<br />
following blastocyst cryopreservation using super-cooling<br />
ultra-rapid vitrification. Hum Reprod 20, <strong>12</strong>2-<strong>12</strong>8. Epub 2004<br />
Oct 2007.<br />
Hunter JE, Fuller BJ, Bernard A et al. 1995 Vitrification of human<br />
oocytes following minimal exposure to cryoprotectants;<br />
initial studies on fertilization and embryonic development.<br />
Hum Reprod 10, 1184-1188.<br />
Isachenko V, Montag M, Isachenko E et al. 2005 Aseptic<br />
technology of vitrification of human pronuclear oocytes<br />
using open-pulled straws. Hum Reprod 20, 492-496. Epub<br />
2004 Nov 2004.<br />
Isachenko V, Selman H, Isachenko E et al. 2003 Modified<br />
vitrification of human pronuclear oocytes: efficacy and effect<br />
on ultrastructure. Reproductive Biomedicine Online 7, 211-216.<br />
Kasai M, Mukaida T 2004 Cryopreservation of animal and<br />
human embryos by vitrification. Reprod Biomed Online 9, 164-<br />
170.<br />
CONTINUED ON PAGE 38<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
37
ARTICLES<br />
CONTINUED FROM PAGE 37<br />
Katayama KP, Stehlik J, Kuwayama M et al. 2003 High survival<br />
rate of vitrified human oocytes results in clinical pregnancy.<br />
Fertil Steril 80, 223-224.<br />
Kuleshova LL, Lopata A 2002 Vitrification can be more favorable<br />
than slow cooling. Fertil Steril 78, 449-454.<br />
Kuleshova LL, Shaw JM 2000 A strategy for rapid cooling of<br />
mouse embryos within a double straw to eliminate the risk<br />
of contamination during storage in liquid nitrogen. Hum<br />
Reprod 15, 2604-2609.<br />
Kuwayama M, Vajta G, Ieda S et al. 2005a Comparison of open<br />
and closed methods for vitrification of human embryos and<br />
the elimination of potential contamination. Reprod Biomed<br />
Online 11, 608-614.<br />
Kuwayama M, Vajta G, Kato O et al. 2005b Highly efficient<br />
vitrification method for cryopreservation of human oocytes.<br />
Reprod Biomed Online 11, 300-308.<br />
Lane M, Gardner DK 2001 Vitrification of mouse oocytes using a<br />
nylon loop. Molecular Reproduction and Development 58, 342-<br />
347.<br />
Lane M, Schoolcraft WB, Gardner DK 1999 Vitrification of mouse<br />
and human blastocysts using a novel cryoloop containerless<br />
technique. Fertil Steril 72, 1073-1078.<br />
Liebermann J, Tucker MJ 2002 Effect of carrier system on the<br />
yield of human oocytes and embryos as assessed by survival<br />
and developmental potential after vitrification. Reproduction<br />
<strong>12</strong>4, 483-489.<br />
Liebermann J, Tucker MJ 2006 Comparison of vitrification and<br />
conventional cryopreservation of day 5 and day 6<br />
blastocysts during clinical application. Fertil Steril 86, 20-26.<br />
Epub 2006 Jun 2008.<br />
Liebermann J, Tucker MJ, Sills ES 2003 Cryoloop vitrification in<br />
assisted reproduction: analysis of survival rates in > 1000<br />
human oocytes after ultra-rapid cooling with polymer<br />
augmented cryoprotectants. Clin Exp Obstet Gynecol 30, <strong>12</strong>5-<br />
<strong>12</strong>9.<br />
Lucena E, Bernal DP, Lucena C et al. 2006 Successful ongoing<br />
pregnancies after vitrification of oocytes. Fertil Steril 85, 108-<br />
111.<br />
Luyet B. (1970) Physical changes occurring in frozen solutions<br />
during rewarming and melting. In Wolstenholme, G. and M,<br />
O.C. (eds.), The Frozen Cell. J & A Churchill, London, pp. 27-<br />
50.<br />
Martino A, Songsasen N, Leibo SP 1996 Development into<br />
blastocysts of bovine oocytes cryopreserved by ultra-rapid<br />
cooling. Biol Reprod 54, 1059-1069.<br />
Mazur P 1963 Kinetics of water loss from cells at subzero<br />
temperatures and the likelihood of intracellular freezing.<br />
Journal of general physiology 47, 347-369.<br />
Mukaida T, Nakamura S, Tomiyama T et al. 2003 Vitrification of<br />
human blastocysts using cryoloops: clinical out<strong>com</strong>e of 223<br />
cycles. Hum Reprod 18, 384-391.<br />
Mukaida T, Wada S, Takahashi K et al. 1998 Vitrification of<br />
human embryos based on the assessment of suitable<br />
conditions for 8-cell mouse embryos. Hum Reprod 13, 2874-<br />
2879.<br />
Rall WF, Fahy GM 1985 Ice-free cryopreservation of mouse<br />
embryos at -196 degrees C by vitrification. Nature 313, 573-<br />
575.<br />
Reed ML, Lane M, Gardner DK et al. 2002 Vitrification of human<br />
blastocysts using the cryoloop method: successful clinical<br />
application and birth of offspring. J Assist Reprod Genet 19,<br />
304-306.<br />
Selman H, Angelini A, Barnocchi N et al. 2006 Ongoing<br />
pregnancies after vitrification of human oocytes using a<br />
<strong>com</strong>bined solution of ethylene glycol and dimethyl<br />
sulfoxide. Fertil Steril 86, 997-1000. Epub 2006 Sep 1011.<br />
Shaw PW, Bernard AG, Fuller BJ et al. 1992 Vitrification of mouse<br />
oocytes using short cryoprotectant exposure: effects of<br />
varying exposure times on survival. Molecular Reproduction<br />
& Development 33, 210-214.<br />
Sher G, Keskintepe L, Mukaida T et al. 2008 Selective vitrification<br />
of euploid oocytes markedly improves survival, fertilization<br />
and pregnancygenerating potential. Reprod Biomed Online<br />
17, 524-529.<br />
Son WY, Yoon SH, Yoon HJ et al. 2003 Pregnancy out<strong>com</strong>e<br />
following transfer of human blastocysts vitrified on electron<br />
microscopy grids after induced collapse of the blastocoele.<br />
Hum Reprod 18, 137-139.<br />
Stachecki JJ, Garrisi J, Sabino S et al. 2008 A new safe, simple and<br />
successful vitrification method for bovine and human<br />
blastocysts. Reprod Biomed Online 17, 360-367.<br />
Stehlik E, Stehlik J, Katayama KP et al. 2005 Vitrification<br />
demonstrates significant improvement versus slow freezing<br />
of human blastocysts. Reprod Biomed Online 11, 53-57.<br />
Szell A, Zhang J, Hudson R 1990 Rapid cryopreservation of<br />
sheep embryos by direct transfer into liquid nitrogen<br />
vapour at -180 degrees C. Reproduction, Fertility, &<br />
Development 2, 613-618.<br />
Takahashi K, Mukaida T, Goto T et al. 2005 Perinatal out<strong>com</strong>e of<br />
blastocyst transfer with vitrification using cryoloop: a 4-year<br />
follow-up study. Fertil Steril 84, 88-92.<br />
Teramoto S, Uchiyama K, Aono F, et al. 2004 The efficacy of<br />
selected single embryo transfer (SET) with vitrification.<br />
Fertil Steril 82, S207.<br />
Vajta G, Holm P, Kuwayama M et al. 1998 Open pulled straw<br />
(OPS) vitrification: a new way to reduce cryoinjuries of<br />
bovine ova and embryos. Molecular Reproduction and<br />
Development 51.<br />
Vanderzwalmen P, Bertin G, Debauche C et al. 2003 Vitrification<br />
of human blastocysts with the Hemi-Straw carrier:<br />
application of assisted hatching after thawing. Hum Reprod<br />
18, 1504-1511.<br />
Vanderzwalmen P, Bertin G, Debauche C et al. 2002 Births after<br />
vitrification at morula and blastocyst stages: effect of<br />
artificial reduction of the blastocoelic cavity before<br />
vitrification. Hum Reprod 17, 744-751.<br />
Wininger JD, Kort HI 2002 Cryopreservation of immature and<br />
mature human oocytes. Semin Reprod Med 20, 45-49.<br />
Wu J, Zhang L, Wang X 2001 In vitro maturation, fertilization<br />
and embryo development after ultrarapid freezing of<br />
immature human oocytes. Reproduction <strong>12</strong>1, 389-393.<br />
Yoon TK, Kim TJ, Park SE et al. 2003 Live births after vitrification<br />
of oocytes in a stimulated in vitro fertilization-embryo<br />
transfer program. Fertil Steril 79, 1323-1326.<br />
38 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
Coda ® Air Technology<br />
Coda ® VOC Filtration Technology for Laboratories, Incubators and Gas Lines<br />
Coda ® – the only Proven VOC Filtering Technology for ART<br />
Coda Tower ® ECO TM<br />
GECO-009/115<br />
GECO-009/230<br />
Coda Tower ® ECO TM & Lo-Boy Filter Kits<br />
GESA-001<br />
Coda® Canister ECO TM Semi-Annual Kit<br />
GEAK-001 . . . . . . . .Coda® Canister ECO TM Annual Kit<br />
CCLS-082 . . . . . . . .Coda® Canister Lo-Boy Semi-Annual Kit<br />
CCLA-082 . . . . . . . .Coda® Canister Lo-Boy Annual Kit<br />
Coda ® Unit<br />
GCIU-010<br />
Coda ® Lo-Boy<br />
GXTU-042/115<br />
GXTU-042/230<br />
Why Should You Use Coda ® <br />
Coda ® Aero<br />
GCAU-010/115<br />
GCAU-010/230<br />
Coda ® Aero Filters<br />
GCAF-001 . . . . .Pkg of 1<br />
GCAF-002 . . . . .Pkg of 2<br />
Coda ® SP<br />
GCSP-010<br />
Coda ® 2 Kit<br />
C2KT-107<br />
C2KT-106 (w/o Coda® Xtra Inline® Filter)<br />
As you know your specimens spend all their time inside your incubators and its standalone<br />
micro-environment. They are subject to unique levels of contaminants, Volatile<br />
Organic Contaminants (VOCs), Chemical Air Contaminants (CACs), vapors, solvents,<br />
micro-organisms, endotoxins, bacteria, viruses and particulates. The air inside incubators<br />
contains 6 times the contaminants of the outside air. Installing a Coda ® inside your<br />
incubator creates a perfectly controllable air environment and constantly circulates the<br />
air and removes VOCs such as Styrene, Acetone, Benzene, Toluene, Octane, n-Decane,<br />
Freon, aldahydes, Nonane, Methylcyclohexane and Butane present in most incubators<br />
and laboratory environment.<br />
The following are some of the <strong>com</strong>mon sources of air contamination inside incubators<br />
and laboratories in general.<br />
• Everytime you open an incubator the air contaminants such as VOCs and<br />
particulates present in any laboratory environment will enter and reside inside<br />
your incubator.<br />
• The in<strong>com</strong>ing incubator gas lines such as CO2, N2 , or trigas contain VOCs and<br />
particulates that may contaminate your incubator.<br />
• All Dishes or plasticware placed inside your incubator release high levels of<br />
styrene and other VOCs.<br />
• Equipment and furniture off-gassing. For example, aldehydes are released from<br />
formica materials.<br />
• New incubator materials release VOCs. New incubators have especially high<br />
levels of VOCs which you can smell easily. This will impact culture results in a<br />
new incubator.<br />
• Unexpected contamination in the air surrounding the building or the laboratory<br />
sourced from general outside air pollution such as car exhausts, industry,<br />
packaging and insulation, construction, adhesives and paints, demolition, and<br />
waste will unexpectedly penetrate into the laboratory and the incubator.<br />
Coda ® has been used successfully worldwide, and has been proven to reduce the<br />
contamination levels of most gasses by up to 500% and in some cases virtually eliminate<br />
unwanted VOCs.<br />
FDA 510(k) Cleared ISO13485:2003 and ISO9001:2000<br />
Coda ® Xtra Inline ® Filters<br />
GILX-001 . . . . . . .Pkg of 1<br />
GILX-006 . . . . . . .Pkg of 6<br />
GILX-0<strong>12</strong> . . . . . . .Pkg of <strong>12</strong><br />
Coda ® Inline ® Filters<br />
GILF-001 . . . . . . .Pkg of 1<br />
GILF-006 . . . . . . .Pkg of 6<br />
GILF-0<strong>12</strong> . . . . . . .Pkg of <strong>12</strong><br />
Coda ® Filters<br />
GCIF-001 . . . . .Pkg of 1<br />
GCIF-006 . . . . .Pkg of 6<br />
GCIF-0<strong>12</strong> . . . . .Pkg of <strong>12</strong><br />
Coda ® Passthru Device<br />
GCPT-050
ARTICLES<br />
Successful vitrification in a closed carrier device of blastocysts<br />
originating from infertile patients, egg donors, or in-vitro maturation<br />
by Lopez J 1 , Zech NH 2 , Frias P 1 , Vanderzwalmen P 2,3<br />
1 IVF Center, Cochabamba, Bolivia<br />
2 IVF Centers Prof. Zech, Bregenz, Austria<br />
3 Centre Hospitalier Inter Regional Cavell (CHIREC), Braine l’Alleud - Bruxelles, Belgium<br />
Vitrification in reduced cooling conditions<br />
Vitrification is a cryopreservation procedure by which<br />
solutions are converted into a glass-like amorphous solid<br />
free of any crystalline structures. In order to reduce the<br />
likelihood of lethal ice crystal formation during transit<br />
through the crystalline phase, open embryo carrier<br />
devices allowing direct contact of the biological sample<br />
with liquid nitrogen were designed (Vajta and Nagy,<br />
2006). This allows extremely high cooling rates (more<br />
than 20.000 C°/min), instantaneously bringing the<br />
embryos below the glass transition temperature where<br />
intra and extracellular parts are captured in an amorphous<br />
state. The advantage of ultra-rapid cooling is that a<br />
vitrified state is obtained even if embryos are exposed to<br />
high concentrations of cryoprotectant solutions for only a<br />
very short period of time, long enough to permit the<br />
protection of the biological material. Similarly, extremely<br />
high warming rates (> 20,000 C°/min) can be achieved,<br />
preventing recrystallization.<br />
However, a major drawback of such ultra-rapid<br />
cooling procedure is the possible risk of bacterial or viral<br />
contamination of the biological sample during cooling and<br />
during long-term storage (Bielanski, 2005). Even though<br />
the question of contamination during storage in liquid<br />
nitrogen (LN2) remains debatable (Kyuwa et al., 2003), it<br />
was important to modify the strategy and develop a<br />
vitrification technique ensuring total protection and<br />
isolation of the sample from LN2 during cooling<br />
procedure and long-term storage. Stachecki et al. (2008)<br />
developed a vitrification technique of blastocysts in closed<br />
0.25 ml straws. After warming, a survival rate of 89% was<br />
obtained and, out of 43 transfers, clinical pregnancy and<br />
implantation rates of 60% and 45%, respectively, were<br />
obtained.<br />
Consequently, a hermetically closed carrier device<br />
(VitriSafe, VitriMed, Austria) that is easy to handle and<br />
guarantees the medical safety of vitrified human embryos<br />
and oocytes was developed. This closed system is a<br />
modification of the original open hemi-straw plug carrier<br />
device (Vanderzwalmen et al., 2003). The VitriSafe<br />
consists of a large gutter in which a small quantity of<br />
cryoprotectant containing the blastocysts can be deposited<br />
(Figure 1) Before plunging the biological material into<br />
LN2, the VitriSafe is <strong>com</strong>pletely inserted into a high<br />
security 0.3 ml straw (CBS,<br />
Cryo Bio System, France).<br />
Both ends of the outer<br />
protective are heat-sealed<br />
before being plunged into<br />
LN2, ensuring hermetic<br />
isolation of the sample. The<br />
VitriSafe guarantees high<br />
warming rates of >20,000 PIERRE VANDERZWALMEN, PHD<br />
C°/min,<br />
without<br />
<strong>com</strong>promising aseptic conditions during extraction from<br />
the outer protective straw.<br />
The protective straw, which is required to achieve<br />
aseptic vitrification conditions, inevitably leads to an<br />
important loss in the cooling rate (~1300 C°/min),<br />
increasing the possibility of ice crystal formation if the<br />
intra-cellular concentration of cryoprotectant is not well<br />
adapted to the need of the cells. Because the probability of<br />
fixing the intracellular parts into a glass-like state depends<br />
on the rate of cooling and re-warming, and the<br />
concentration of cryoprotectant solutions (Yavin et al.,<br />
2007), it is obvious that the risk of ice crystal formation will<br />
increase if the cells have not been exposed long enough to<br />
the cryoprotectant solutions. Consequently, the step of<br />
exposure of the embryo to the cryoprotectant solutions<br />
before cooling at a lower rate, has to be considered as a<br />
crucial one. In fact, the decrease in the rate of cooling as<br />
observed with the VitriSafe device has to be <strong>com</strong>pensated<br />
for by longer exposure to the cryoprotectant solutions in<br />
order to increase the intra-cellular concentration.<br />
In order to determine the optimal concentration of the<br />
cryoprotectant solutions and the duration of contact to<br />
these solutions, a microscopic cinematographic evaluation<br />
was undertaken. This study showed how embryos react<br />
during the dehydration and entrance steps with different<br />
cryoprotectant concentrations and times of exposure to the<br />
solutions. The morphometric analysis permitted us to<br />
determine the shrinkage/swelling response of embryos to<br />
increased concentrations of cryoprotectant and thereby<br />
stay within the limits of volume variation that are<br />
<strong>com</strong>patible with good survival rate. When a longer<br />
exposure to the cryoprotectant solutions is required, a<br />
gradual exposure in three steps induces less shrinkage –<br />
swelling stress to the cell than does a two-step addition.<br />
40 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
Figure 1: The use of the VitriSafe system for the vitrification of human embryos. a) the VitriSafe embryo carrier (G = gutter),<br />
b) embryos in the gutter of the VitriSafe embryo carrier (CPS = cryoprotectant solution), c) a 0.3 ml CBS straw, d) the VitriSafe embryo<br />
carrier inserted in a 0.3 ml hermetically closed CBS straw before plunging into LN2.<br />
Clinical application<br />
The protocol that we have devised consists of a<br />
gradual exposure of the embryos to 5%-5%, 10%-10% and<br />
20%-20% DMSO-ethylene-glycol (EG) before aseptic<br />
vitrification using the VitriSafe carrier (Table 1).<br />
As shown in Table 2, this approach resulted in<br />
satisfactory clinical out<strong>com</strong>es of 233 aseptic blastocyst<br />
vitrification/warming cycles despite reduced cooling rates<br />
to
ARTICLES<br />
CONTINUED FROM PAGE 41<br />
Table 1. The procedure for vitrification and warming of<br />
blastocysts with the VitriSafe device.<br />
Vitrification<br />
Step 1 5% DMSO/5% EG 5 to 10 min<br />
Step 2 10% DMSO/10% EG 4 min<br />
Step 3 20% DMSO/20% EG 40 to 60 sec<br />
Warming<br />
Step 1 Plunge the tip of the Vitrisafe in 1 ml of 1.0 M<br />
sucrose for 1 min 30 sec.<br />
Step 2 0.75 M Sucrose 1 min 30 sec<br />
Step 3 0.50M Sucrose 2 min<br />
Step 4 0.25M Sucrose 2 - 3 min<br />
Step 5 0.<strong>12</strong>5M Sucrose 2 - 3 min<br />
Culture<br />
Embryo culture in Global medium (LifeGlobal, Ontario, Canada)<br />
It is well accepted that lower implantation rates are<br />
seen after a fresh embryo transfers with IVM embryos as<br />
<strong>com</strong>pared to standard IVF/ISCI. One of the reasons might<br />
be the sub-optimal quality of the endometrium. The<br />
challenge with IVM is to retrieve immature oocytes from<br />
small antral follicles – usually in mid to late follicular<br />
phase, and to avoid the occurrence of dominant follicles.<br />
The endometrium is exposed to relatively low levels of<br />
estradiol when immature oocytes are retrieved from the<br />
small antral follicles. This explains the reduced thickness<br />
of the endometrium. Additionally, embryos are placed<br />
back into the uterus much earlier than after normal<br />
IVF/ICSI with respect to the number of days that the<br />
endometrium encounters increasing estradiol levels.<br />
Taken together, with IVM and fresh ET, a synchrony<br />
between the endometrium and embryos has to take place<br />
in a greatly accelerated time schedule <strong>com</strong>pared to other<br />
types of ART. For these reasons, one alternative to fresh<br />
embryo transfer could be the cryopreservation of embryos,<br />
with subsequent transfers in an estradiol/progesterone<br />
supplemented cycle.<br />
Ongoing twin pregnancy after vitrification of<br />
blastocysts produced after IVM from a woman with PCOS<br />
was reported by Son et al. (2002). To the best of our<br />
knowledge, this is the first report of pregnancies from a<br />
large series of vitrified blastocysts produced from IVM<br />
oocytes retrieved from PCOS women.<br />
Compared to the transfers of IVM-derived embryos in<br />
fresh cycles, vitrification in the VitriSafe system resulted in<br />
a significant increase in the implantation rate. A study was<br />
undertaken to <strong>com</strong>pare the out<strong>com</strong>e of IVM cycles after<br />
embryo transfers in fresh cycles or after vitrification and<br />
Table 2. Clinical out<strong>com</strong>es of aseptic vitrification of blastocysts generated from different origins.<br />
Origin of blastocysts Male and/or Egg donation IVM Total<br />
female factor<br />
infertility<br />
Number of patients 103 91 22 216<br />
Patient age (years, mean + SD) 34.5 + 3.7 28.8 +3.1 32.3 + 4.9<br />
Number of vitrification – warming cycles <strong>12</strong>0 91 22 233<br />
Number of vitrified blastocysts 348 427 64 839<br />
Number of warmed blastocysts 348 218 64 630<br />
Survival after warming 285 (82%) 203 (93%) 53 (83%) 541 (86%)<br />
Survival before embryo transfer 254 (73%) 191 (88%) 44 (69%) 489 (78%)<br />
Number of embryo transfers 111 91 21 223<br />
Number of blastocysts transferred (mean) 231 (2.1) 186 (2.0) 39 (1.9) 456 (2.0)<br />
Pregnancies (per vitrification – warming cycle) 66 (55%) 54 (59%) 15 (68%) 135 (58%)<br />
Miscarriages 15 (23%) 7 (13.0%) 4 (27%) 26 (19%)<br />
Ongoing pregnancies (per vitrification – warming cycle) 51 (43%) 48 (53%) 11 (50%) 110 (47%)<br />
Number of fetal heart beats 60 64 15 139<br />
Implantation rate 26% 34% 38% 30%<br />
Deliveries 14 35 5<br />
42 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
replacement in an estradiol/progesterone<br />
supplementation transfer cycle. A total of 34 fresh transfers<br />
and 49 vitrified transfers were performed. The ongoing<br />
pregnancy rates in the fresh and vitrified group were 21%<br />
and 44%, respectively, with implantation rates of 10 and<br />
30%.<br />
In spite of reduced cooling rates due to aseptic<br />
vitrification conditions, acceptable results are obtainable if<br />
the intracellular concentrations of cryoprotectants are well<br />
adapted to the needs of the cells. If such results are<br />
confirmed on a large scale, aseptic vitrification has the<br />
potential to be<strong>com</strong>e the standard cryopreservation<br />
technique, not only for human blastocysts but also for<br />
early embryo developmental stages.<br />
References<br />
Bielanski A 2005 Non-transmission of bacterial and viral<br />
microbes to embryos and semen stored in the vapour phase<br />
of liquid nitrogen in dry shippers. Cryobiology 50, 206-210.<br />
Kyuwa S, Nishikawa T, Kaneko et al. 2003 Experimental<br />
evaluation of cross-contamination between cryotubes<br />
containing mouse 2-cell embryos and murine pathogens in<br />
liquid nitrogen tanks. Experimental Animal 52, 67-70.<br />
Son WY, Yoon SH, Park SJ, et al., 2002 Ongoing twin pregnancy<br />
after vitrification of blastocysts produced by in-vitro<br />
matured oocytes retrieved from a woman with polycystic<br />
ovary syndrome: case report. Human Reproduction; 17:2963-<br />
2966.<br />
Stachecki J, Garrisi J, Sabino S et al., (2008) A new safe, simple<br />
and successful vitrification method for bovine and human<br />
blastocysts. Reproductive BioMedicine Online 17, 360-367.<br />
Vanderzwalmen P, Bertin G, Debauche CH et al. 2003<br />
Vitrification of human blastocysts with the Hemi-Straw<br />
carrier: application of assisted hatching after thawing.<br />
Human Reproduction 18, 1504-1511.<br />
Vanderzwalmen P, Ectors F, Grobet L, et al., 2009, Development<br />
of an aseptic vitrification technique: application to<br />
blastocysts originating from infertile patients, egg donors<br />
and after in vitro maturation. Reproductive BioMedicine<br />
Online. (in press).<br />
Vajta G, Nagy Z 2006 Are programmable freezers still needed in<br />
the embryo laboratory Review on vitrification. Reprod<br />
BioMed Online 7, 623-633.<br />
Yavin S, Arav A 2007 Measurement of essential physical<br />
properties of vitrification solutions. Theriogenology 67, 81-89.<br />
You can contact Pierre Vanderzwalmen, PhD at: pierrevdz@hotmail.<strong>com</strong>.<br />
A unique PGD Biopsy Medium formulation<br />
based on global ® medium<br />
PGD Biopsy Medium is ready-to-use and designed to maintain embryos during<br />
the biopsy procedure, allowing ease in removal of blastomeres.<br />
PGD Biopsy Medium was designed along with the staff of Reprogenetics, led by<br />
Santiago Munné, in conjunction with our ongoing development of global ® based<br />
on Simplex Optimization and the entire line of LifeGlobal ® media.<br />
embryo GPS ®<br />
– The best dish for PGD cases<br />
PGD Biopsy Medium Advantages<br />
• PGD Biopsy Medium is calcium- and magnesium-free to break the<br />
cadherin bonds between the blastomeres and thereby allow for the<br />
removal of one or two blastomeres for PGD.<br />
• PGD Biopsy Medium contains sucrose to cause a mild shrinkage of the<br />
blastomeres and thereby facilitate removal of the blastomere(s) for PGD.<br />
• PGD Biopsy Medium contains all the other <strong>com</strong>ponents of global ®<br />
embryo culture medium including energy substrates and amino acids.<br />
This reduces the stress on the embryo and promotes better development<br />
of the embryo after it is returned to culture.<br />
• PGD Biopsy Medium is HEPES-buffered for use outside of a CO 2<br />
incubator, and contains gentamicin and HSA so that it is ready to use.<br />
• PGD Biopsy Medium was designed in consultation with Santiago<br />
Munné and his scientific staff at Reprogenetics, one of the foremost PGD<br />
laboratories in the world.<br />
www.LifeGlobal.<strong>com</strong><br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
43
ARTICLES<br />
Life-long impact of the first cell cycle<br />
by Dmitri Dozortsev, MD, PhD<br />
Advanced Fertility Center of Texas, Houston, TX, USA, American College of Embryology<br />
Whether you believe that life begins at conception<br />
or not, the life of each and every one of us began<br />
at the pronuclear stage, when two mortal cells<br />
came together and transcended into the continuum of<br />
immortality of humans as a species. There are many<br />
critical stages in the development of a human being but<br />
the first cell cycle stands out in its significance, ever more<br />
so as we advance our knowledge about it. In this brief<br />
article I will discuss the three most crucial events of the<br />
first cell cycle, and how they may be affected by<br />
embryology practitioner now, and into the future.<br />
First, during natural fertilization, the development is<br />
set into motion by calcium ion waves elicited by a factor in<br />
the sperm (1), most likely phospholipase C-zeta (PLCzeta)<br />
(2), located initially in the head of a spermatozoon<br />
and subsequently migrating into the pronucleus.<br />
Experimental evidence suggests that full term<br />
development is also possible following parthenogenetic<br />
activation (3). Those studies, and Ozil’s work (4) in<br />
particular, clearly demonstrate that the duration and<br />
amplitude of calcium oscillations affect embryonic<br />
developments after implantation. On the other hand, from<br />
clinical experience with sharing of donor eggs, we have<br />
learned that embryonic development is affected by a<br />
fertilizing spermatozoon. Therefore, it is plausible that<br />
even though the activation itself is all or nothing<br />
phenomenon, the amount of PLC-zeta carried by a given<br />
sperm cell could affect the duration and amplitude of<br />
oscillations and thus produce an effect similar to that<br />
observed in Ozil’s experiments. In the same way that<br />
intracytoplasmic sperm injection turned out to be more<br />
effective than natural fertilization, we may one day find<br />
that artificial activation is more efficient and allows more<br />
control over the activation process than natural activation.<br />
The second crucial event involves the telomeres. There<br />
is accumulating evidence of the importance of the length<br />
of the telomeres for the life span, probability of cancer, and<br />
the length of reproductive life. Because it has been well<br />
established that the length of the telomeres shortens with<br />
every cell cycle due to inability of telomerase to replicate<br />
in 5’-3’ direction (5), it is obvious that at some point during<br />
reproduction length of the telomeres must be restored.<br />
However, until recently, the stage and mechanism of this<br />
restoration remained a <strong>com</strong>plete mystery. It was not until<br />
2007 that this puzzle was solved. It turned out that<br />
telomere length abruptly increases during pronuclei stage<br />
by a telomerase independent mechanism through sister<br />
chromatid exchange (6).<br />
This is the only<br />
opportunity to reset the<br />
length of the telomeres in<br />
an individual’s lifetime. It<br />
is possible that a better<br />
understanding of how<br />
telomere length is restored<br />
may allow us to extend and<br />
improve life span. Only the<br />
embryology practitioner<br />
will be in a position to<br />
DMITRI DOZORTSEV, MD, PHD<br />
ac<strong>com</strong>plish that.<br />
Furthermore, we must<br />
realize that we may already be unknowingly affecting this<br />
process, and will have to wait for a few more decades to<br />
see if our in-vitro interventions are affecting life span.<br />
The repair of DNA damage is the third critical event<br />
happening during the first cell cycle. By the time of<br />
fertilization, both gametes, particularly the oocyte, have<br />
accumulated some damage. Of particular concern is DNA<br />
damage by by-products of oxidative phosphorylation,<br />
which may result in as many as 1 million individual<br />
molecular lesions per day. Data suggest that, in the oocyte,<br />
the telomeric region of the chromosomes is particularly<br />
vulnerable, leading to chromosomal non-disjunctions, the<br />
majority of which take place during meiosis I (7).<br />
In the sperm, no regional preference for chromosomal<br />
damage has been clearly demonstrated, while there is<br />
overwhelming evidence that sperm cells accumulate a<br />
very significant damage to their DNA, particularly in cases<br />
with abnormal sperm parameters. Unlike the oocyte, the<br />
spermatozoon has virtually no mechanism to repair DNA<br />
damage. Thus, one of the critical missions of the first cell<br />
cycle is to repair the DNA from both gametes. The<br />
accuracy requirements for this repair are much higher<br />
than for any other cell, as it will serve as the template for<br />
all other cells. Reparative DNA synthesis must precede<br />
DNA replication. If the damage was extensive, it may<br />
increase the length of the first cell cycle. In any other cell,<br />
this increase would not matter much, because of the<br />
checkpoint that prevents premature activation of p34<br />
kinase, which does not begin phosphorylation of histones<br />
before the DNA is replicated. However, during the first<br />
cell cycle cell, division is not as tightly coupled with<br />
chromosomal replication as it is in any other cell. In fact,<br />
experiments show that the enucleated zygote will undergo<br />
44 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
cell division, albeit disorganized, around the time when it<br />
would normally take place. Furthermore, an experiment<br />
with okadaic acid (OA) clearly demonstrated uncoupling<br />
between <strong>com</strong>pletion of DNA replication and nuclear<br />
envelope break down (8). The exact mechanism for that is<br />
unknown. One hypothesis states that OA specifically<br />
activates H1 kinase, although it is more plausible that it<br />
simply gives advantage to H1 kinase, removing a<br />
counterbalancing influence of a phosphatase. However,<br />
whatever the mechanism, remarkably it only forces the<br />
zygote into premature chromosome condensation, but<br />
does not affect 2-cell or any later-stage embryos. These<br />
and other observations suggest that the normal progress<br />
of the first cell cycle relies, at least<br />
in part, on a general balance of phosporylation/<br />
dephosphorylation, and that shifting it one way or the<br />
other will delay or accelerate its pace. One of the factors<br />
that has a powerful impact on this balance is the pH of the<br />
culture medium, which is in turn affected by CO2<br />
concentration, specific for each culture medium.<br />
Therefore, ‘playing’ with pH gives us a unique<br />
opportunity to control the first cell cycle in a multitude of<br />
ways. Beyond the cell cycle, it is likely to also impact DNA<br />
methylation, responsible for gene imprinting, which was<br />
shown recently, may differ drastically between in-vitro<br />
and in-vivo generated embryos (9). The significance of this<br />
is to be understood in the future.<br />
In summary, from the moment of fertilization, our<br />
future existence is molded by epigenetic pressures which<br />
<strong>com</strong>e in the variety of forms. Therefore, embryology<br />
practitioners are to a large extent, responsible for<br />
establishing a strong foundation for the future of the<br />
human being that may be born as a result of their efforts.<br />
synthesis of polynucleotides and biological significance of<br />
the phenomenon. J. Theor. Biol. 41: 181-90<br />
6. Liu, Lin; Bailey, Susan M; Okuka, Maja; Munoz, Purificacion;<br />
Li, Chao; Zhou, Lingjun; Wu, Chao; Czerwiec, Eva; Sandler,<br />
Laurel; Seyfang, Andreas; Blasco, Maria A; Keefe, David L.<br />
(2007) Telomere lengthening early in development. Nature<br />
Cell Biol.; 9: 1436-41<br />
7. Keefe, D L; Liu, L; Marquard, K. (2007) Telomeres and agingrelated<br />
meiotic dysfunction in women. Cell. Mol.Life Sci: 64:<br />
139-43<br />
8. Dyban AP, De Sutter P, Verlinsky Y. (1993) Okadaic acid<br />
induces premature chromosome condensation reflecting the<br />
cell cycle progression in one-cell stage mouse embryos. Mol<br />
Reprod Dev 34:402-15.<br />
9. Gomes MV, Huber J, Ferriani RA, Amaral Neto AM, Ramos<br />
ES. (2009) Abnormal methylation at the KvDMR1<br />
imprinting control region in clinically normal children<br />
conceived by assisted reproductive technologies. Mol Hum<br />
Reprod 15:471-7.<br />
You can contact Dmitri Dozortsev at: dmitrid385@hotmail.<strong>com</strong><br />
References<br />
1. Dozortsev D, Rybouchkin A, De Sutter P, Qian C, Dhont M.<br />
(1995) Human oocyte activation following intracytoplasmic<br />
injection: the role of the sperm cell. Hum Reprod. 10:403-7.<br />
2. Parrington J, Swann K, Shevchenko VI, Sesay AK, Lai FA.<br />
(1996) Calcium oscillations in mammalian eggs triggered by<br />
a soluble sperm protein. Nature. 379(6563):364-8.<br />
3. Rybouchkin AV, Van der Straeten F, Quatacker J, De Sutter P,<br />
Dhont M. (1997) Fertilization and pregnancy after assisted<br />
oocyte activation and intracytoplasmic sperm injection in a<br />
case of round-headed sperm associated with deficient<br />
oocyte activation capacity. Fertil Steril. 68:1144-7.<br />
4. Ozil JP (1990) The parthenogenetic development of rabbit<br />
oocytes after repetitive pulsatile electrical stimulation..<br />
Development 109:117-27.<br />
5. Olovnikov AM. (1973) A theory of marginotomy. The<br />
in<strong>com</strong>plete copying of template margin in enzymic<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
45
ARTICLES<br />
Automated Robotic Human ICSI<br />
Navid Esfandiari, DVM, PhD, HCLD 1 ; Zhe Lu, PhD2; Xuping Zhang, PhD 2 ; Robert F. Casper, MD 1 ; Yu Sun, PhD 2<br />
1 Toronto Centre for Advanced Reproductive Technology (TCART), Department of Obstetrics and Gynecology, and<br />
2 Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, Canada.<br />
Before the introduction of micromanipulation, the<br />
majority of cases of severe male infertility were<br />
generally not treatable. In-vitro fertilization (IVF)<br />
cycles were cancelled in about one-third of these cases,<br />
due to failed fertilization. The introduction of<br />
micromanipulation techniques such as zona drilling,<br />
partial zona dissection, and subzonal insemination<br />
reduced the incidence of failed fertilization and salvaged<br />
many IVF cycles. However, there was little improvement<br />
on fertilization and pregnancy out<strong>com</strong>e because these<br />
procedures required a relatively high number of<br />
progressive motile sperm which in turn frequently<br />
resulted in a high incidence of polyspermy in inseminated<br />
eggs. To over<strong>com</strong>e these limitations, microinjection of a<br />
single sperm into the ooplasm (intracytoplasmic sperm<br />
injection, ICSI) was developed. ICSI resulted in a dramatic<br />
improvement in fertilization rates and revolutionized the<br />
treatment of infertile couples with severe male factor.<br />
ICSI, however, is a labor-intensive laboratory<br />
procedure and is technically challenging. Manual<br />
microinjection and micromanipulation of human oocytes<br />
requires a long period of training, and is further limited<br />
by low speed, human errors, and inter-operator<br />
variability. Although ICSI is now considered routine, it<br />
remains a very difficult technique to master, due partly to<br />
its inherent technical difficulty and partly to the<br />
heterogeneity of the oocytes. It is generally agreed that the<br />
ICSI procedure is subject to a learning curve and that not<br />
depositing the spermatozoon within the oocyte cytoplasm<br />
is a <strong>com</strong>mon technical failure.<br />
The degeneration of oocytes after ICSI is often a result<br />
of a fault in the ICSI technique, for example an injection<br />
pipette that is either too large or not sharp enough. Proper<br />
orientation of the polar body and needle position are also<br />
important, because improper positioning can damage or<br />
disrupt the metaphase plate during needle entry.<br />
Moreover, disturbances in the nuclear spindle may<br />
dispose oocytes to aneuploidy or maturation arrest. Thus,<br />
perturbation of the oocyte cytoskeleton may critically<br />
influence the fate of the embryo. During ICSI, the location<br />
of the first polar body is <strong>com</strong>monly used as an indication<br />
of the spindle position, with the assumption that they are<br />
located in close proximity. To avoid damage to the spindle,<br />
oocytes are injected at the 3 o’clock position with the first<br />
polar body at the 6 or <strong>12</strong> o’clock position.<br />
NAVID ESFANDIARI, DVM, PHD, HCLD<br />
The skill of the embryologist conducting the ICSI<br />
procedure is considered a significant predictor of<br />
fertilization, whereas laboratory conditions (i.e.<br />
incubators, culture of oocytes individually versus<br />
grouped, etc.) do not affect the rates as much. The lack of<br />
automated systems capable of high-throughput human<br />
oocyte injection hinders both clinical practice and<br />
research. The goal of our present research is to develop a<br />
robotic system that can quickly and reproducibly inject a<br />
single sperm into the cytoplasm of a human oocyte, or<br />
manipulate the oocyte/zygote.<br />
At present, the automated ICSI system <strong>com</strong>prises the<br />
following elements:<br />
• a standard inverted microscope (Bright field<br />
imaging, 20X objective, Nikon Ti-S)<br />
• a CMOS camera (601f, Basler)<br />
• an in-house developed vacuum-based cell holding<br />
device for immobilizing multiple oocytes<br />
• an in-house developed precision vacuum pump<br />
• an in-house developed motorized rotational stage<br />
placed on a motorized X-Y translational stage<br />
(ProScan, Prior Scientific Inc.) for oocyte<br />
positioning and orientation control<br />
• a straight ICSI micropipette (MIC-50-0,<br />
Humagen) connected to a 25 microliter glass<br />
syringe (Hamilton) filled with mineral oil and<br />
mounted on a linear stage (eTrack, Newmark<br />
System Inc.) for <strong>com</strong>puter-controlled sperm<br />
aspiration and deposition<br />
• a three-dimensional motorized micromanipulator<br />
(MP285, Sutter Inc.) for positioning the ICSI<br />
micropipette (45° tilting angle) to diagonally<br />
penetrate the oocyte<br />
• a heated stage (THN-60-10, LINKAM) to maintain<br />
oocytes and sperm at 37°<br />
46 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
• a host <strong>com</strong>puter for controlling multiple motion<br />
control devices and processing images in real time<br />
• a vibration isolation table (9100 series, KSI)<br />
• a dissecting microscope (SZX <strong>12</strong>, Olympus) for<br />
loading oocytes and sperm onto the cell holding<br />
device.<br />
The procedure begins with loading sperm and oocytes<br />
onto the cell holding device under the dissecting<br />
microscope and the culture medium is covered with<br />
mineral oil. A low vacuum holds an oocyte on top of each<br />
through-hole in the cell holding device. The cell holding<br />
device is then transferred onto the rotational stage on the<br />
inverted microscope. The first oocyte is positioned at<br />
center of the microscope field by moving the X-Y<br />
translational stage. A straight ICSI micropipette is lowered<br />
until the tip of the micropipette roughly appears in the<br />
image. The system integrates a vision-based contact<br />
detection algorithm to automatically determine the vertical<br />
position of the micropipette tip and device surface in the<br />
oocyte area. The micropipette is then automatically moved<br />
to the sperm area to perform contact detection in order to<br />
determine the vertical position of the micropipette tip and<br />
device surface in the sperm area. The position of the sperm<br />
area is also recorded by the system. To inject a sperm into<br />
an oocyte, the user first selects a sperm by mouse clicking<br />
the sperm head on the monitor of the host <strong>com</strong>puter<br />
(Figure 1).<br />
Figure 1: The monitor of the host <strong>com</strong>puter<br />
CONTINUED ON PAGE 48<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
47
ARTICLES<br />
CONTINUED FROM PAGE 47<br />
The system automatically tracks the motion of the<br />
sperm and taps its tail to immobilize it. The user then<br />
aspirates the sperm into the micropipette through the<br />
control program interface. When a sperm is positioned in<br />
the proximity of micropipette opening, an oocyte is<br />
automatically brought into the field of view. The user<br />
identifies the position of the polar body on the monitor,<br />
and, if needed, the system automatically rotates the polar<br />
body away from the penetration site. The system performs<br />
penetration, sperm deposition, and micropipette<br />
retraction all through <strong>com</strong>puter control. For the next<br />
injection, the system moves the sperm area into the field of<br />
view for the user to select the next sperm for injection.<br />
This process is repeated until all oocytes have been<br />
injected. At the end of ICSI, the cell holding device is taken<br />
off the rotational stage and put under the dissecting<br />
microscope. A low positive pressure is applied to release<br />
the oocytes for collection and transfer to proper culture<br />
dishes for further culture.<br />
In our preliminary study we aimed to quantify the<br />
efficiency of the robotic microinjection system using<br />
mouse zygotes. The robotic ICSI system was first tested to<br />
inject one-cell mouse embryos with phosphate buffered<br />
saline. The embryos were cultured in the same condition<br />
(KSOM, 37°C, 5% CO 2 ) as the control group. The system<br />
demonstrated an injection speed of <strong>12</strong> mouse zygotes per<br />
minute; a lysis rate of 1.1%; and a high blastocyst<br />
formation rate of 89.8% which was similar to the control<br />
group.<br />
The system is currently being tested by injecting<br />
human sperm into hamster oocytes before moving on to<br />
clinical ICSI evaluation. Figure 2 shows a sperm being<br />
injected into a hamster oocyte by the robotic system. We<br />
expect the automated system to produce a high success<br />
rate and consistency in human ICSI, which, together with<br />
other features such as skill independence, and short<br />
learning curve, will make the system a useful tool for<br />
clinical ICSI practice.<br />
References:<br />
1. Esfandiari N, Javed M, Gotlieb L, Casper RF. (2005)<br />
Complete failed fertilization after intracytoplasmic sperm<br />
injection - analysis of 10 years data. Int J Fertil Women’s Med<br />
50:187-92.<br />
2. X.Y. Liu and Y. Sun (2009) Automated mouse embryo<br />
injection moves toward practical use. IEEE International<br />
Conf. on Robotics and Automation (ICRA2009), Kobe, Japan,<br />
May <strong>12</strong>-17.<br />
3. Javed M, Esfandiari N, Casper RF. (2010) Failed fertilization<br />
after intracytoplasmic sperm injection. Reprod. Biomed. Online<br />
20: 56-67.<br />
You can contact Navid Esfandiari at: navid.esfandiari@utoronto.ca<br />
Figure 2: Hamster ICSI: (A) held hamster oocyte, (B, C) a human sperm is being injected into a hamster oocyte<br />
48 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
AN ALTERNATE METHOD FOR SHIPPING SPERM: AEROGEL<br />
CONTAINERS FITTED WITH DATA LOGGERS<br />
By Lakshmi Sharma M.Sc, M.Phil, ELD ., Susan Tarchala M.S, T.S ., Jon Nakagawa* CPA<br />
John Perry * B.S, MBA ., Richard Rawlins PhD, HCLD.<br />
Rush Center for Advanced Reproductive Care, Chicago, Illinois<br />
*Marathon Products, San Leandro, CA<br />
Temperature maintenance and documentation play a<br />
vital role in the IVF laboratory. Media and sperm<br />
samples used in the laboratory must be stored and<br />
shipped within specific temperature ranges in order to<br />
maintain the integrity of the products and to produce<br />
optimal end results. Dry shippers charged with liquid<br />
nitrogen are the current method of choice for shipping<br />
sperm, and they are safe and ensure temperature<br />
maintenance at -196 C for up to 5 days. However, dry<br />
shippers are bulky and typically weigh around 20 lbs<br />
when fully charged. Freight costs for these vessels average<br />
between $200 and $250 for 2-day delivery within the<br />
continental United States, and this cost is passed down to<br />
the patient. We propose an alternative method for<br />
shipping sperm to the laboratory using carbon based<br />
aerogel containers that are filled with dry ice.<br />
Aerogels are produced by extracting the liquid<br />
<strong>com</strong>ponent of a gel through supercritical drying and<br />
replacing it with a gas. This allows the liquid to be slowly<br />
drawn off without causing the solid matrix in the gel to<br />
collapse from capillary action, as would happen with<br />
conventional evaporation (Pierre and Pajonk, 2002).<br />
Aerogels are good thermal insulators because they almost<br />
nullify three methods of heat transfer (convection,<br />
conduction and radiation) (Fricke and Emmerling, 1992).<br />
For example, NASA uses aerogel for thermal insulation of<br />
the Mars Rover and space suits.<br />
(http://stardust.jpl.nasa.gov/tech/aerogel.html).<br />
A modification of this material to produce boxes that<br />
are then filled with dry ice and can maintain temperature<br />
of -70 to -80 C for extended time periods have been used<br />
in this study. Documentation of maintenance of cold chain<br />
is ensured by a data logging device, the microDL<br />
(Marathon Products, San Leandro CA). Figure 2.<br />
containing 1.5 cc of<br />
cryopreserved sperm<br />
samples frozen in EYB<br />
(Irvine Scientific) by<br />
conventional methods were<br />
taken out of liquid nitrogen<br />
storage and placed onto the<br />
dry ice. The flat ribbon-like<br />
probe of the microDL was<br />
LAKSHMI SHARMA, MSC, MPHIL, ELD<br />
placed next to the vials and<br />
secured in place by taping it to the walls of the box. The<br />
cryovials were then covered with another 0.5 lbs of dry ice<br />
so the sperm samples lay sandwiched between the 2 layers<br />
of dry ice. The entire box was placed into a standard<br />
corrugated shipping box lined with Styrofoam. One box<br />
was labeled as day 3 and the other day 5. The final weight<br />
of the boxes ranged from 2.0 to 2.5 lbs. The microDL was<br />
programmed to measure temperature inside the aerogel<br />
containers every 4 minutes. The temperature data from the<br />
microDL units were downloaded on to a micro<strong>com</strong>puter.<br />
We used both internal and external controls for this<br />
study. Internal controls were cryopreserved sperm in 3.0<br />
ml Nunc vials prepared for shipping in a standard fully<br />
charged MVE dry shipper, and external controls were<br />
sperm received from a <strong>com</strong>mercial cryobank.<br />
Post thaw motility and forward progression on sperm<br />
samples was measured on day 3 and day 5. The box<br />
labeled day 3 was opened on the third day and post thaw<br />
motility and forward progression noted and <strong>com</strong>pared to<br />
controls in the dry shipper. Similarly, the box labeled as<br />
day 5 was opened the fifth day and post thaw motility and<br />
forward progression noted and <strong>com</strong>pared to controls.<br />
Materials and Methods<br />
Two aerogel boxes (Figure 1b) were prepared for<br />
shipping in the following manner. The boxes were placed<br />
overnight in a freezer and pre-equilibrated to -10 C prior<br />
to being filled with 0.5 lbs of dry ice. Nunc cryovials<br />
CONTINUED ON PAGE 50<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
49
ARTICLES<br />
CONTINUED FROM PAGE 49<br />
Figure 1a: Carbon based aerogel boxes.<br />
Figure 1b: Carbon based aerogel boxes prepared for shipping<br />
with dry ice.<br />
Figure 2: Micro dl data loggers<br />
Results<br />
The results of temperature measurements from the<br />
microDL showed that the temperature inside the boxes<br />
was maintained between -70 and -80 C, as long as the box<br />
was unopened for a maximum period of 5 days (Figure 3).<br />
The box was opened on day 3 and the samples evaluated<br />
for post thaw sperm motility and forward progression<br />
which were <strong>com</strong>parable to the control. The box opening<br />
resulted in rapid rise of the internal temperature to reach<br />
room temperature within 24 hrs. A similar pattern of in the<br />
rising of temperature after the box was opened on day 5<br />
was seen. Post thaw motility and forward progression of<br />
the samples day 3 and day 5 were <strong>com</strong>parable controls<br />
(Table 1). Poor post-thaw viability was noted for frozen<br />
thawed 1-cell mouse embryos on both day 3 and day 5,<br />
using this method.<br />
50 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
Table 1: Sperm motility and forward progression on Day 3 and Day 5. 1cell mouse embryo viabilty post thaw.<br />
Internal Control External Control<br />
Dry Shipper Cryobank Sample 1 Sample 2<br />
Day 3 percent motility 62% 64% 60% _<br />
Day 3 forward progression 61% 60% 54% _<br />
D5 percent motility 63% _ _ 61%<br />
Day 5 forward progression 61% _ _ 56%<br />
1 cell mouse embryos – 19/21 6/21 6/20<br />
Viability at thaw (90.4%) (28.5%) (30%)<br />
Figure 3: Temperature data on day 3 and day 5.<br />
Discussion and Conclusions<br />
In conclusion the carbon based aerogel boxes provide<br />
a viable alternative to liquid nitrogen dry shippers<br />
without <strong>com</strong>promising the post thaw quality of semen<br />
samples over a short time period of 3 to 5 days but are not<br />
optimal for mouse embryo shipping. It has previously<br />
been shown that sperm can be stored in dry ice for short<br />
period of time with relatively modest loss of motility (CRC<br />
handbook of laboratory diagnosis and treatment of<br />
fertility, Brooks A. Keel and Bobby W. Webster page 235)<br />
The microDL unites provide an added measure of quality<br />
assurance documenting the maintenance of the cold chain.<br />
This method is also cost effective; the container weighs 2-<br />
2.5 lbs, and freight costs are one-tenth of that for a dry<br />
shipper, and their lighter weight makes them easier to<br />
handle.<br />
References<br />
Pierre A. C. and Pajonk G. M. (2002). Chemistry of aerogels and<br />
their applications. Chemical Reviews 102: 4243-4266.) .<br />
Fricke, J and Emmerling, A (1992). Aerogel – preparation,<br />
properties, applications. Structure & Bonding 77:37-87.<br />
You can contact Lakshmi Sharma at: lakshmisharma63@aol.<strong>com</strong><br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
51
ARTICLES<br />
Epigenetics and the ART Lab<br />
by Sangita Jindal, PhD, HCLD<br />
Montefiore’s Institute for Reproductive Medicine and Health, Albert Einstein College of Medicine Hartsdale, NY, USA<br />
The concept of epigenetics has been discussed with<br />
greater frequency in genetics literature in recent<br />
years. This brief article summarizes the most up-todate<br />
understanding of the connection between epigenetics<br />
and assisted reproductive technologies (ART).<br />
Genomic imprinting is a modification of the genome<br />
in which genes from only one, rather than two, parental<br />
alleles are expressed. Epigenetics is the mechanism by<br />
which genomic imprinting occurs. A sex-specific mark or<br />
imprint on certain chromosomal regions has been<br />
identified for approximately 200 genes in the human<br />
genome (Luedi et al., 2007). In these regions, the paternal<br />
and maternal genomes are functionally non-equivalent,<br />
differing in DNA methylation or histone modification of<br />
the genome, rather than in alterations of DNA sequences.<br />
These stable but reversible epigenetic modifications<br />
determine whether genes in those regions of the genome<br />
are expressed.<br />
During development, only specific sets of genes are<br />
active. Accessibility of transcription factor binding sites on<br />
DNA is determined by specific patterns of chromatin<br />
modification and DNA methylation. Chromatin exists in a<br />
transcriptionally <strong>com</strong>petent or transcriptionally silent<br />
state. For example histone acetylation is typically<br />
associated with rendering transcription binding sites<br />
<strong>com</strong>petent while histone methylation renders them silent.<br />
We know that sweeping changes in DNA methylation<br />
and chromatin remodeling take place in developing germ<br />
cells and in the pre-implantation embryo, rendering them<br />
particularly vulnerable to environmentally induced<br />
epigenetic modifications. Epigenetics in the germ line has<br />
been most extensively studied in the mouse, in which the<br />
primary imprints are established during gametogenesis.<br />
This is followed by a second wave of epigenetic erasure<br />
after fertilization with asymmetric demethylation of the<br />
male (active) and female (passive) pronuclei. Finally, a<br />
third wave of de-novo methylation of the embryonic<br />
genome occurs at the blastocyst stage (Grace and Sinclair,<br />
2009; Laprise, 2009).<br />
Of the 200 imprinted genes in the human genome,<br />
approximately 50 have been associated with a phenotype<br />
or clinical syndrome (Amor and Halliday, 2008). Of those,<br />
a link has most <strong>com</strong>monly been suggested between ART<br />
procedures and Beckwith-Wiedemann Syndrome (BWS)<br />
and Angelman Syndrome (AS). BWS is characterized by<br />
prenatal overgrowth, umbilical hernia and increased risk<br />
of developing tumors, and AS is characterized by severe<br />
developmental delay,<br />
absent speech and<br />
hypotonia. These<br />
syndromes both involve<br />
an epigenetic defect of<br />
hypomethylation on the<br />
maternal allele of the<br />
relevant differentially<br />
methylated regions. The<br />
incidence of BWS in the<br />
general population is 0.8% SANGITA JINDAL, PHD, HCLD<br />
but BWS occurred in 4.6%<br />
of the ART babies examined in the study by (DeBaun et al.,<br />
2003). Studies have yielded inconsistent results but<br />
together they suggest that epigenetic errors may originate<br />
from specific aspects of ART such as the reason for the<br />
subfertility itself, ovulation induction, use of IVF or ICSI,<br />
and finally in-vitro culture and embryo transfer.<br />
One theory proposes that there exist built-in buffers<br />
that prevent every mutation from being expressed as a<br />
phenotype; if these buffers are removed, hidden genetic<br />
variation within a population can be phenotypically<br />
expressed (Horsthemke and Ludwig, 2005). ARTconceived<br />
babies usually develop normally, although ART<br />
bypasses many biological filters such as selective gamete<br />
resorption, selective sperm uptake and sperm <strong>com</strong>petition.<br />
ART also introduces many environmental stresses to the<br />
gametes and embryos such as exogenous hormones<br />
during ovulation induction, chemical and pH fluctuations<br />
in culture media, lab fluctuations in temperature,<br />
humidity, light, and air quality. Evidence suggests that<br />
maturational or environmental changes caused by ART,<br />
both in the ovary and in the lab, have greater effects on the<br />
oocyte than on the sperm (Niemitz and Feinberg, 2004).<br />
Khosla et al. (2001) showed that serum addition to culture<br />
media changed the methylation pattern of imprinted<br />
genes in mouse embryos, and was related to a reduction in<br />
fetal birth weight. It has been postulated that the absence<br />
of the amino acid methionine in media may be involved in<br />
the hypo-methylation of genes during the preimplantation<br />
phase of mouse embryo culture (Niemitz and Feinberg,<br />
2004), and Menezo (2006) stresses the importance of<br />
including methionine in embryo culture medium at all<br />
stages of development.<br />
Follow up studies of ART-conceived babies are<br />
reassuring, but many studies are difficult to interpret due<br />
to small sample size, absence of correction for etiology of<br />
52 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
infertility or maternal age, and the reliance on selfreporting<br />
surveys (Laprise, 2009). As a result, there<br />
remains a lack of reliable data on relative and overall risk<br />
of rare imprinting syndromes in ART-conceived children.<br />
Further study is needed to detect subtle phenotypes<br />
affecting health, growth, behavior, predisposition to<br />
disease, all of which may be partially due to aberrant<br />
imprinting. Imprinting may have a wider impact on<br />
neurological development and behavior. Some reports<br />
suggest parent-specific imprinting defects are involved in<br />
childhood expression of autism, bipolar disorder,<br />
schizophrenia, alcohol abuse and audiogenic seizures<br />
(Paoloni-Giacobino and Chaillet, 2004).<br />
In conclusion, it is clear that the extracellular<br />
environment during critical periods of development is a<br />
key mechanism underlying epigenetic modifications<br />
(Thompson et al., 2002). In-vitro culture and manipulation<br />
in the laboratory can alter oocyte and embryo cell<br />
physiology by stress-induced cellular responses such as<br />
production of reactive oxygen species and cytokines, and<br />
can therefore lead to altered early gene expression<br />
through epigenetic mechanisms. Given the strong but<br />
circumstantial evidence linking an increased risk of<br />
epigenetic syndromes to ART, long term follow up in welldefined<br />
studies on children conceived from ART are<br />
re<strong>com</strong>mended.<br />
References<br />
Amor, D.J., Halliday, J. (2008) A review of known imprinting<br />
syndromes and their association with assisted reproduction<br />
technologies. Hum Reprod 23, 2826-2834.<br />
DeBaun, M.R., Niemitz, E.L., Feinberg, A.P. (2003) Association of<br />
in vitro fertilization with Beckwith-Wiedemann syndrome<br />
and epigenetic alterations of LIT1 and H19. Am J Hum Genet<br />
72, 156-160.<br />
Grace, K.S., Sinclair, K.D. (2009) Assisted reproductive<br />
technology, epigenetics, and long-term health: a<br />
developmental time bomb still ticking. Semin Reprod Med 27,<br />
409-416.<br />
Horsthemke, B., Ludwig, M. (2005) Assisted reproduction: the<br />
epigenetic perspective. Hum Reprod Update 11, 473-482.<br />
Laprise, S.L., 2009, Implications of epigenetics and genomic<br />
imprinting in assisted reproductive technologies. Mol Reprod<br />
Dev 76, 1006-1018.<br />
Luedi, P.P., Dietrich, F.S., Weidman, J.R., Bosko, J.M., Jirtle, R.L.,<br />
Hartemink, A.J. (2007) Computational and experimental<br />
identification of novel human imprinted genes. Genome Res<br />
17, 1723-1730.<br />
Menezo, Y. (2006) Paternal and maternal factors in<br />
preimplantation embryogenesis: interaction with the<br />
biochemical environment. Reprod BioMed Online <strong>12</strong>, 616-621.<br />
Niemitz, E.L., Feinberg, A.P. (2004) Epigenetics and assisted<br />
reproductive technology: a call for investigation. Am J Hum<br />
Genet 74, 599-609.<br />
Paoloni-Giacobino, A., Chaillet, J.R. (2004) Genomic imprinting<br />
and assisted reproduction. Reprod Health 1, 6.<br />
Thompson, J.G., Kind, K.L., Roberts, C.T., Robertson, S.A.,<br />
Robinson, J.S. (2002) Epigenetic risks related to assisted<br />
reproductive technologies: short- and long-term<br />
consequences for the health of children conceived through<br />
assisted reproduction technology: more reason for caution<br />
Hum Reprod 17, 2783-2786.<br />
You can contact Sangita Jindal at: sangita.k.jindal@gmail.<strong>com</strong><br />
The Tri-Vac for aspiration of oocyte…<br />
Pioneer Pro-Pumps are the Most Relaible Aspiration Pump<br />
Used in IVF<br />
• Quietest in the industry • Responsive<br />
• Light Weight<br />
• 5 Year Warranty<br />
• Vacuum 0 to 300 mmHg • Made in USA<br />
• ISO 9001:2000 • ISO 13485:2003<br />
• Steel Housing<br />
• 110V & 220V<br />
• CE Registered<br />
• FDA 510(k) Cleared<br />
Ordering Information<br />
Pioneer TriVac . . . . .PTPP-010/115<br />
Accessory Kit . . . . . .PTAK-010<br />
www.IVFonline.<strong>com</strong><br />
US/Canada: 1-800-720-6375 • International: 1-519-826-5800 • Fax: 1-519-826-6947 • email: sales@IVFonline.<strong>com</strong><br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
53
ARTICLES<br />
Improving air quality in ART laboratories<br />
by Giles Palmer<br />
ESHRE Senior Clinical Embryologist, Director of Assisted Conception Unit, Mitera Hospital, Athens, Greece<br />
Air in urban areas can contain high levels of<br />
pollutants such as carbon monoxide, nitrous<br />
oxide, sulphur dioxide, and heavy metals.<br />
Indoors, construction materials, MDF, PVC flooring,<br />
paints and adhesives are major sources of volatile organic<br />
<strong>com</strong>pounds (VOCs) leading to the “sick building<br />
syndrome”. Other sources of indoor chemical hazards are<br />
cleaning fluids, floor waxes, cosmetics and cigarette<br />
smoke.<br />
National health and safety authorities set safe limits of<br />
VOC exposure for humans and give guidelines for<br />
building ventilation-but there is little evidence in the IVF<br />
literature of the toxicological effect of these on embryos<br />
in vitro.<br />
Pollutants can settle on work surfaces and dissolve in<br />
aqueous solutions of embryo culture medium. Embryos,<br />
lacking an immune system, cannot protect themselves<br />
against these environmental contaminants.<br />
Cohen et al. (1997) illustrated that high levels of<br />
aldehydes and other noxious <strong>com</strong>pounds are present not<br />
only in the IVF laboratory (higher than outside air and the<br />
average house), but also in the incubators. Controlling air<br />
quality in an ART laboratory has shown beneficial effects<br />
on fertilization and embryo development (Boone et al.,<br />
1999).<br />
In Europe the EU directive 2004/23/EC, which<br />
stipulates quality requirements when Human tissue and<br />
cell are handled (a critical point being clean air), has led to<br />
most centers making, at the very least, slight structural<br />
changes to their IVF units. The implementation of the<br />
directive has created problems in the IVF industry, some<br />
measures being contradictory to temperature control<br />
associated with ART procedures, but do not mention<br />
reduction of damaging <strong>com</strong>pounds in the air such as<br />
VOCs.<br />
Laboratory design<br />
If one is fortunate and can design an IVF facility from<br />
the beginning, then the most important consideration<br />
should be its location. The challenges of reducing air<br />
pollution should be factored into your initial design<br />
phase.<br />
Control of the air quality may be hindered in an urban<br />
area, or in units close to busy roads and car parks. Within<br />
a hospital, difficulties may be encountered with working<br />
in an area adjacent to the laundry, sterilizing or histology<br />
departments.<br />
If embryo development<br />
and implantation depends<br />
so highly on its culture<br />
environment, the IVF<br />
laboratory should do well<br />
to follow the stringent<br />
regulatory standards of the<br />
food and pharmaceutical<br />
industry.<br />
Clean room technology<br />
GILES PALMER<br />
creates a carefully<br />
controlled sealed environment in which the number of<br />
particles and contamination is significantly minimized.<br />
This is achieved using highly filtered air under positive<br />
pressure being flushed through High Efficiency Particle<br />
Air (HEPA) filters. All furniture, cleaning methods and<br />
clothing must be appropriate for use in a clean room,<br />
consistent with the level of air quality required.<br />
The laboratory should have smooth, non-porous<br />
walls, impervious unbroken surfaces with no difficult-toclean<br />
corners and ledges, no sliding doors, sinks and<br />
drains. The lighting should be within sealed units.<br />
Partition wall systems specially designed for clean rooms<br />
or made from a non-porous material such as Corian® are<br />
expensive but provide an inert, hypoallergenic, easy-toclean<br />
wall surface. Ducts and pipes can be hidden between<br />
wall panels or covered within an alcove to prevent dust<br />
accumulation.<br />
Traditionally copper pipes have been used to transport<br />
the gas from cylinder to incubator. Copper, however, is<br />
prone to oxidation and therefore inert stainless steel<br />
tubing, certified for use with medical gasses, is now<br />
re<strong>com</strong>mended.<br />
Egg collection and embryo transfer involve<br />
cooperation with operating theatre, and this can creates a<br />
challenge for maintaining clean air conditions.<br />
Hermetically-sealed doors and pass- through windows<br />
may help these critical steps.<br />
Changing rooms should be as clean as possible,<br />
flushed with filtered air, and adjacent to the laboratory.<br />
Positive pressure in the laboratory will be easier to<br />
maintain and not require such a high velocity output if the<br />
adjoining operating theater also has sealed doors and<br />
ceiling.<br />
Provision should be given to how bulky ART<br />
equipment will access the unit after the construction<br />
phase. A removable sealed section of wall allows not only<br />
54 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
ventilation in the final stages of building but an easy way<br />
to bring large equipment such as incubators and laminar<br />
flow cabinets into the <strong>com</strong>pleted laboratory space.<br />
New equipment<br />
Whenever possible, new incubators should be “run<br />
in” in a room other than the laboratory for a certain time<br />
period to dissipate latent VOCs produced in their<br />
manufacture.<br />
This may make the difference between a poor initial<br />
pregnancy rate and a triumphant start to a new IVF unit!<br />
How can an existing laboratory improve its air quality<br />
Other than install a central air handling system that<br />
includes activated carbon pre-filters and HEPA filters,<br />
several <strong>com</strong>mercial mobile filter units exists which clean<br />
air quite efficiently- purifying the air of damaging volatile<br />
<strong>com</strong>pounds, bacteria and moulds (Forman et al., 2004). Air<br />
is forced through the device and either passed through<br />
carbon filters and/or ultra violet light for photo catalytic<br />
purification.<br />
Compressed gas can contain harmful <strong>com</strong>pounds<br />
such as benzene, isopropanol and pentane (Cohen et al.,<br />
1997). Medical grade O 2 and CO 2 can be filtered before<br />
entering the incubators by gas line filters. Because high<br />
levels of VOCs have been shown to be present in<br />
incubators, internal air filtering systems may be added to<br />
decrease VOC levels inside incubators themselves. (Hall et<br />
al., 1998., Mayer et al., 1999).<br />
Anesthetic gasses used in oocytes retrieval may linger<br />
in the air, and therefore a suitable extraction system may<br />
be installed in the operation theatre.<br />
Renovation and building work<br />
Renovating an existing laboratory can introduce a<br />
variety of <strong>com</strong>pounds and emission of harmful<br />
<strong>com</strong>pounds used in finishing can have an adverse effect<br />
on embryo development and pregnancy rate either<br />
temporarily or long term! Paints, adhesives and sealants<br />
can contain alkanes, aromatics, alcohols, aldehydes and<br />
ketones, among others. Epoxy paints emit VOCs and take<br />
several weeks to cure, and thus their use should be<br />
avoided.<br />
When renovating an older laboratory, there are<br />
materials that can be used with less harmful chemicals:<br />
Solvent-free adhesives used for floor covering are<br />
available with low VOC emissions, as well as “ecological”<br />
water based paints that contain non volatile chemicals.<br />
On site supervision is essential to assure no materials<br />
or methods are used that could jeopardize later IVF<br />
success.<br />
Removal of wooden furniture<br />
To reduce VOC emissions from laboratory shelves,<br />
cupboards and tables can be replaced with stainless<br />
furniture and workbenches, as used in the pharmaceutical,<br />
electronic, and food industries.<br />
Access<br />
Entry of unauthorized personnel, packaging, and<br />
other materials into the laboratory should be restricted.<br />
An air lock chamber before entering the laboratory offers<br />
both a physical barrier and acts as an "air shower" if<br />
flushed with filtered air.<br />
Laboratory dress code<br />
Staff can be one of the biggest contaminants in a clean<br />
environment. Clean room clothing should provide<br />
maximum control against microbial and particulate<br />
contamination. Cotton hospital “greens” should be<br />
replaced by pocket-less trouser suits, or two piece suits<br />
with covered wrists and neck. Made specifically for clean<br />
rooms, these polyester garments act as particle barriers<br />
and shed no fibers into the environment.<br />
Purging<br />
Enough time to purge the room of harmful chemicals<br />
should be allocated before laboratory start up. For at least<br />
for one week, room-temperature and positive air pressure<br />
should be increased to hasten the removal of VOCs<br />
introduced during construction.<br />
We renovated our laboratory in Athens in August<br />
2009, following the above measures. The laboratory<br />
CONTINUED ON PAGE 56<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
55
ARTICLES<br />
CONTINUED FROM PAGE 55<br />
premises were already fitted with activated-carbon pre<br />
filters, HEPA filters, and in-line filters, but an upgrade to<br />
a clean room GMP Class C (ISO class 7) was sanctified.<br />
Completion took one month and VOC measurements<br />
of the environment using ACS badges were measured<br />
during each critical phase of reconstruction and the<br />
following two weeks. The highest VOC levels were<br />
observed in the adhesion of Corian© wall panels where<br />
acetone reached a level of 15.5 ppm. Vinyl glue used in the<br />
final stages of floor surfacing also indicated emissions of<br />
hazardous VOCs.<br />
One week of purging was performed after<br />
<strong>com</strong>pletion. Two weeks after <strong>com</strong>pletion, no detectable<br />
chemicals were present. Figure 1 illustrates the VOC<br />
emissions in various phases before and after renovation.<br />
Figure 1: Chemicals detected during renovation of the ART<br />
laboratory.<br />
better environment for the culture of oocytes and<br />
embryos. It is up to every IVF facility to access the clean air<br />
requirements and determine if changes need to be made.<br />
Maintaining good air quality<br />
To maintain the clean air environment, good<br />
laboratory practice must be continued. Frequent<br />
measurements of air flow, and servicing of equipment<br />
safeguards against a drop in air quality due to exhausted<br />
HEPA filters.<br />
Positive pressure between the laboratory and adjacent<br />
rooms can be monitored by the pressure differential<br />
recorded daily to indicate filter efficiency.<br />
Validation of air quality can be measured periodically<br />
by particle counters, and VOC levels should be monitored<br />
either electronically, or using inexpensive organic<br />
chemical sensors that analyze absorbed ion levels on<br />
chromatography paper which can measure workplace<br />
exposure of toxic vapors.<br />
Agar contact plates or <strong>com</strong>mercially available kits are<br />
used to count microbial load on surfaces. Establishing<br />
bench marks for conventional in-vitro fertilization rate,<br />
embryo quality, and pregnancy rates adds to your total<br />
quality management.<br />
References<br />
Although not statistically significant, an improvement<br />
of IVF out<strong>com</strong>e was observed after renovation of our<br />
clinic in Athens (Table 1). This reassures us that the<br />
reconstruction of the laboratory did not have a<br />
detrimental effect on our assisted conception program,<br />
and an adequate air filtration system was possibly already<br />
in place. We are pleased, however, with our new working<br />
environment and are satisfied that we have created a<br />
W.R. Boone, J.E. Johnson, A. Locke, M.M. Crane, T.M. Price<br />
(1997). Control of air quality in an assisted reproductive<br />
technology laboratory. Fertil. Steril. 71, 150-154.<br />
J. Cohen, A. Gilligan, W. Esposito, T. Schimmel, B. Dale (1997).<br />
Ambient air and its potential effects on conception in vitro.<br />
Hum. Reprod. <strong>12</strong>, 1742-1749.<br />
M. Forman, V. Polanski, A. Gilligan, D. Rieger. (2004) Reduction<br />
in volatile organic <strong>com</strong>pounds, adehydes, and particulate<br />
air contaminants in an IVF laboratory by centralized and<br />
stand alone air filtration systems. Fertil Steril. 82, Suppl.2. P-<br />
535 (Abstract).<br />
J. Hall, A. Gilligan, T. Scchimmel, M. Cecchi, J. Cohen (1998). The<br />
origin, effects and control of air pollution in laboratories for<br />
human embryo culture. Hum. Reprod. 13, 146-155.<br />
J.F. Mayer, F. Nehchiri, V.M Weedon et al. (1999) Prospective<br />
randomized crossover analysis of the impact of an IVF<br />
incubator air filtration system (Coda. Gen X) on clinical<br />
pregnancy rates. Fertil Steril. 72, Suppl. 1. S42.<br />
You can contact Giles Palmer at: gpalmer@mitera.gr<br />
Table 1: Pregnancy and implantation rates before (May to July) and after (September to November) renovation of the ART laboratory.<br />
May June July September October November<br />
No. Transfers 97 76 74 54 96 98<br />
No. Positive β-HCG 37 (38%) 32 (42%) 24 (32%) 22 (41%) 55 (57%) 53 (54%)<br />
Clinical Pregnancy rate 25 (26%) 18 (24%) 14 (19%) 15 (27%) 27 (28%) 37 (38%)<br />
Implantation rate 39 (16%) 30 (17%) 19 (11%) 25 (18%) 53 (22%) 64 (24%)<br />
56 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
The original Coda Tower ® has been redesigned to SAVE ENERGY<br />
and to HELP our environment, giving you the new Coda Tower ®<br />
ECO TM .<br />
The ECO TM has a smaller footprint, using less laboratory space, while<br />
increasing air flow, CFM’s and air exchanges, changing the air in your<br />
laboratory more times per minute. More air exchanges means cleaner air.<br />
Our Coda ® Canister filters remove and reduce the levels of VOCs, (volatile<br />
organic contaminants) and CACs (chemical air contaminants) while our 99.997%<br />
HEPA filter removes far more particulates due to the increased air exchanges.<br />
Benefits of the Coda ® Tower ECO TM<br />
ECO-friendly ECO-nomical ECO-filter pack<br />
ISO13485:2003 and ISO9001:2000 Company<br />
FDA 510(k) Cleared<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
email: sales@IVFonline.<strong>com</strong>
ARTICLES<br />
The use of birefringence technology in<br />
assisted human reproduction<br />
by Simon J Phillips, MSc, BSc<br />
OVO Fertility, 8000 Boulevard Decarie #100, Montréal, Québec H4P 2S4, Canada<br />
Introduction<br />
Polarization technology is capable of visualizing<br />
highly ordered structures, since these structures split the<br />
polarized light beam, change the plane of vibration, and<br />
retard the polarised light. This effect is called<br />
birefringence. Such highly ordered structures found in<br />
gametes include the meiotic spindle, the inner and outer<br />
layers of the zona pellucida, and sperm heads. The use of<br />
birefringence technology in ART was first suggested by<br />
Wang et al (2000), although the filamentous nature of the<br />
spindle observed by birefringence was first noted by<br />
Inoué (1953). By using alternating perpendicularorientated<br />
polarising and analyser lenses, nonbirefringent<br />
structures will remain dark. A birefringent<br />
structure will be<strong>com</strong>e visible if the <strong>com</strong>pensator optics are<br />
rotated so that the polarised light that has been changed in<br />
its plane of vibration by the object can pass through. The<br />
limiting factor had been that conventional polarized light<br />
microscopy can assess only relatively large differences in<br />
changes of retardance and biological structures have<br />
relatively little birefringence. Orientation-independent<br />
polarizing microscopy (PolScope) uses circularly<br />
polarized light and an electronically controlled liquid<br />
crystal analyser to assess objects. In <strong>com</strong>bination with a<br />
CCD camera and specific software, this permits the user to<br />
analyse small changes in the region of a few nanometres<br />
associated with biological samples.<br />
Using a PolScope, Wang et al (2000) assessed the<br />
meiotic spindle of oocytes to ensure maturation prior to<br />
ICSI being used. Since then, studies have been published<br />
proposing the use of the PolScope to the morphology in<br />
terms of the oocyte spindle and the zona pellucida.<br />
Applications including the selection of embryos for<br />
transfer, assuring the integrity of cryopreserved/thawed<br />
oocytes for ICSI and assessing the location of the meiotic<br />
spindle for ICSI have been suggested. (Cohen et al., 2004;<br />
De Santis et al., 2005; Kilani et al., 2006). Retardance<br />
measurements in addition to meiotic spindle length and<br />
azimuth can be recorded using the PolScope, as well as<br />
spindle location and physical evidence of a poorly formed<br />
spindle. (Figure 1)<br />
The following data are from two studies; prospective<br />
data collection using the PolScope in stimulated IVF<br />
cycles, and using the PolScope in controlled, natural cycle<br />
IVF.<br />
SIMON J PHILLIPS, MSC, BSC<br />
Materials and Methods<br />
Subjects<br />
All patients presenting during the test period for<br />
either stimulated or controlled natural cycle IVF and<br />
having need of ICSI were included in the study.<br />
Clinical Protocol<br />
Our clinical protocol for controlled natural cycle IVF<br />
has been described previously. (Phillips et al., 2007;<br />
Kadoch et al., 2007). Ovarian stimulation for stimulated<br />
IVF cycles was performed using standard GnRH agonist<br />
long protocols, GnRH antagonist protocols, or GnRH<br />
agonist short microdose protocols, depending on the<br />
patient profile and the physician choice.<br />
Laboratory Protocol<br />
At the time of ICSI, all oocytes were assessed using the<br />
Oosight (CRI, USA) to evaluate the position of the meiotic<br />
spindle in the oocyte. ICSI was performed at 38-40 hours<br />
post-hCG. In addition an image was taken using the<br />
software associated with the Oosight which allows for<br />
analysis of various measurement parameters of the<br />
meiotic spindle once the oocyte has been returned to the<br />
incubator.<br />
Results<br />
A total of 11<strong>12</strong> metaphase II oocytes from stimulated<br />
IVF cycles were assessed using the PolScope. In 81.6% of<br />
oocytes, it was possible to visualize the spindle. The<br />
pregnancy rate and clinical pregnancy rate were 51% and<br />
40% respectively. (Table 1)<br />
When looking at data from stimulated IVF cycles and<br />
<strong>com</strong>paring those cycles resulting in a pregnancy with<br />
cycles not resulting in a pregnancy, there were 68 pregnant<br />
cycles and 66 non pregnant cycles. There were no<br />
significant differences in the number of assessed oocytes<br />
58 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
Figure 1: Meiotic spindle (top left) at ICSI assessed using the Cri Oosight system. It is clear that the microfilaments of the spindle have<br />
not formed correctly, increasing the chance of aneuploidy in the resulting embryo. Indeed this oocyte resulted in an embryo with 2<br />
pronuclei but with a z-score of Z4. (Central image) Previous work (Scott et al 2000) associated an increased risk of aneuploidy with<br />
Z4 pronuclei.<br />
Table 1: Information for all cycles in stimulated cycles<br />
sIVF<br />
Number of cycles 134<br />
Av. Patient age 34.4<br />
Number of oocytes 11<strong>12</strong><br />
Proportion of oocytes with visualised spindle 81.6%<br />
Pregnancy rate / cycle 51%<br />
Clinical pregnancy rate / cycle 40%<br />
CONTINUED ON PAGE 60<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
59
ARTICLES<br />
CONTINUED FROM PAGE 59<br />
or age of patients. (Table 2) There was no difference<br />
between the proportions of visualized spindles in the two<br />
groups. (83.1% versus 79.7%)<br />
Spindle measurements were <strong>com</strong>pared between the<br />
two groups and there was no significant difference<br />
between the average retardance of spindles from<br />
pregnant cycles versus non pregnant cycles for both all<br />
oocytes and also when only the oocytes that resulted in<br />
transferred embryos were <strong>com</strong>pared. However, a trend<br />
towards a difference was noted. (p=0.07, p=0.08<br />
respectively). There was, however, a statistically<br />
significant difference between the two groups for spindle<br />
length. (p=0.02) (Table 3)<br />
In addition the data from controlled natural IVF<br />
cycles was collected; the retardance and spindle length<br />
were <strong>com</strong>pared with those from stimulated IVF cycles.<br />
Forty-eight nIVF cycles were included from the same<br />
study time period. There were no differences seen<br />
between the nIVF and sIVF groups in either spindle<br />
retardance or spindle length. (Table 4)<br />
Discussion<br />
We assessed a large number of oocytes and, in<br />
addition, <strong>com</strong>pared oocytes derived from controlled<br />
natural cycle IVF as well as traditional stimulated IVF<br />
cycles from which previous studies have performed their<br />
evaluations. This allowed us to <strong>com</strong>pare conception cycles<br />
with non-conception cycles and verify whether meiotic<br />
spindle measurements were predictive of out<strong>com</strong>e.<br />
Our data suggest that the spindle length may be<br />
indicative of oocyte <strong>com</strong>petency because there was a<br />
significant difference in spindle length between<br />
conception cycles and non-conception cycles. This<br />
confirms the findings of Ramu Raju et al (2007) whose<br />
looked at embryo development relative to various spindle<br />
parameters and found a correlation between spindle<br />
length and blastocyst rate. However, Kilani et al (2009)<br />
showed shorter spindle length in conception cycles than<br />
in non-conception cycles. This apparent contradiction<br />
may <strong>com</strong>e down to a question of numbers, since their<br />
study, although very well designed, only assessed 22<br />
versus 30 oocytes, whereas we were able to look at over<br />
1000 oocytes.<br />
We saw no significant correlation between spindle<br />
retardance was demonstrated between conception and<br />
Table 2: Standard information of cycles with positive and those with negative pregnancy tests<br />
sIVF Conception cycles Non-conception cycles<br />
Cycles (n) 68 66<br />
Oocytes (n) 620 492<br />
Av. Age 33.5 35.3<br />
Av. no. Eggs 9.1 7.5<br />
Table 3: Comparison of spindle measurements between pregnant and non-pregnancy cycles<br />
sIVF Conception cycles Non-conception cycles P<br />
Av. Retardance -all eggs (sd) 2.11 (0.55) 2.03 (0.61) 0.066<br />
Av. Retardance - transferred eggs (sd) 2.10 (0.57) 1.95 (0.59) 0.08<br />
Av. Spindle Length (sd) 13.2 (1.93) <strong>12</strong>.48 (2.06) 0.015<br />
Table 4: Comparison of spindle measurements between stimulated and controlled natural IVF cycles<br />
nIVF sIVF P<br />
Av. Retardance - transferred eggs (sd) 1.8 (0.64) 2.0 (0.58) NS<br />
Av. Spindle length (sd) <strong>12</strong>.7 (1.95) <strong>12</strong>.6 (2.16) NS<br />
60 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
non-conception cycles, again in contradiction of Kilani et<br />
al. (2009). However, we did see a trend towards increased<br />
spindle retardance in the conception cycles. Furthermore,<br />
the range of retardance values seen for spindles is small<br />
and there is some overlap between the groups, so that it<br />
may be difficult to clearly define limits for ‘good’ and<br />
‘poor’ spindle retardance. In our data, a value of<br />
approximately 2.0 seemed to represent a shift between<br />
these two groups, however other published data have<br />
shown higher values attributable to ‘good’ spindle<br />
retardance. This may be related to system set-up and<br />
therefore may mean that intra-laboratory retardance<br />
values may be of limited value.<br />
No differences were seen between parameters<br />
evaluated in controlled natural cycle IVF derived oocytes<br />
and traditional IVF cycles with ovarian stimulation<br />
derived oocytes. Insufficient numbers in the nIVF group<br />
prevented us from analyzing conception versus nonconception<br />
cycles in this particularly interesting group of<br />
oocytes. These data are currently being prospectively<br />
collected for future analysis.<br />
To date there is increasing evidence of the interest and<br />
possible application of birefringence technology with ART.<br />
However, further research is still required to quantify<br />
those applications.<br />
As research develops with birefringence technology it<br />
may be<strong>com</strong>e a more widely used indicator of oocyte<br />
quality and embryo selection in ART cycles, especially<br />
since it is, importantly, a non-invasive method of<br />
assessment. A recent review of this technology proposed<br />
that it may be a useful tool for aneuploidy research in<br />
oocytes. (Shen et al., 2008) The report of Gianaroli et al<br />
(2008) has suggested that birefringence technology may be<br />
useful in sperm selection for ICSI, and further research is<br />
required to confirm potential benefits to this approach as<br />
well as to explain the reason behind any benefit.<br />
Suggestions that sperm head birefringence could be<br />
related to sperm DNA damage certainly warrant further<br />
investigation. (Damasceno et al., 2008)<br />
References<br />
Cohen Y, Malcov T, Schwartz N et al. (2004) Spindle imaging: a<br />
new marker for optimal timing of ICSI Human Reproduction<br />
19, 649-654.<br />
De Santis L, Cino I, Rabellotti E, et al. (2005) Polar body<br />
morphology and spindle imaging as predictors of oocyte<br />
quality. Reproductive Biomedicine Online. 11, 36-42.<br />
Damasceno-Vieira A, Silva C, Ying Y et al. (2008) A non-invasive<br />
method to assess DNA damage in individual sperm. Fertility<br />
and Sterility 90, Suppl., S11 (abstract O-30).<br />
Gianaroli L, Magli MC, Collodel et al. (2008) Sperm head’s<br />
birefringence: a new criterion for sperm selection. Fertility<br />
and Sterility. 90, 104-1<strong>12</strong><br />
Gianaroli L, Magli MC Ferraretti AP et al. (2008) Birefringence<br />
characteristics in sperm heads allow for the selection of<br />
reacted spermatozoa for intracytoplasmic sperm injection.<br />
Fertility and Sterility. 2008. Epub ahead of print.<br />
Inoué S. 1953. Polarisation optical studies of the mitotic spindle.<br />
1. The demonstration of spindle fibers in living cells.<br />
Chromosoma 5, 487-500.<br />
Kadoch IJ, Al-Khaduri M, Phillips SJ, Lapensee L, Couturier B,<br />
Hemmings R, Bissonnette F. (2007) Spontaneous Ovulation<br />
Rate before Oocyte Retrieval in Controlled Natural Cycle In<br />
Vitro Fertilisation (nIVF) with and without Indomethacin.<br />
Reproductive Biomedicine Online. 16, 245-249.<br />
Kettel LM, Roseff SJ, Chiu TC et al (1991) Follicular arrest during<br />
the midfollicular phase of the menstrual cycle; a<br />
gonadotropin-releasing hormone antagonist imposed<br />
follicular-follicular transition. The Journal of clinical<br />
endocrinology & metabolism. 73, 644-649.<br />
Kilani SS, Cooke S, Kan AK, Chapman MG. (2006) Do age and<br />
extended culture affect the architecture of the zona pellucida<br />
of human oocytes and embryos Zygote. 14, 39-44.<br />
Kilani S, Cooke S, Kan A, Chapman M. (2009) Are there noninvasive<br />
markers in human oocytes that can predict<br />
pregnancy out<strong>com</strong>e Reproductive Biomedicine Online. 18,<br />
674-680.<br />
Phillips SJ, Kadoch IJ, Lapensée L, et al (2007) Controlled natural<br />
cycle IVF: our experience in a world of stimulation.<br />
Reproductive Biomedicine Online. 14, 356-359.<br />
Rama Raju GA. (2007) Meiotic spindle and zona pellucida<br />
characteristics as predictors of embryonic development: a<br />
preliminary study using PolScope imaging. Reproductive<br />
Biomedicine Online. 149,166-174.<br />
Rongières-Bertrand C, Olivennes F, Righini C et al (1999) Revival<br />
of the natural cycles in in-vitro fertilization with the use of a<br />
new gonadotrophin-releasing hormone antagonist<br />
(Cetrorelix): a pilot study with minimal stimulation. Human<br />
Reproduction. 14, 683-688.<br />
Shen L, Betzendahl L, Tinneberg HR, Eichenlaub-Ritter U. (2008)<br />
Enhanced polarizing microscopy as a new tool in<br />
aneuploidy research in oocytes. Genetic Toxicology and<br />
Environmental Mutagenesis. 651, 131-140.<br />
Wang WH, Meng L, Hackett RJ et al. (2001) The spindle<br />
observation and its relationship with fertilisation after<br />
intracytoplasmic sperm injection in living human oocytes.<br />
Fertility and Sterility. 75, 348-353.<br />
You can contact Simon J Phillips at: s.phillips@cliniqueovo.<strong>com</strong><br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
61
• Proven Results<br />
• Over 20 independent studies with published results<br />
• Stringent Quality Control<br />
Figure 1. Development of human embryos cultured in global ® from Day 1 to Day<br />
3 and Day 3 to Day 5, or in G1 from Day 1 to Day 3 and then in G2 from Day 3 to Day<br />
5. The proportion of embryos reaching the blastocyst stage by Day 5 was significantly<br />
great in global ® . The pregnancy and implantation rates were significantly greater<br />
for embroys cultures in global ® than for those cultured in G/G2. (Zech<br />
et al., Human Reprod. 21, Suppl. 1, i162, 2006)<br />
Figure 2. Development of human embryos cultured in global ® from Day 1 to Day 3<br />
and Day 3 to Day 5, or in BAS1 from Day 1 to Day 3 and then in BAS2 from Day 3 to<br />
Day 5. The proportion of embryos having 6 or more cells on Day 3 was significantly<br />
greater in global ® . The blastocyst rate on Day 5 and the pregnancy rate were not<br />
different between media treatments (Matsubara et al., Proc. 24th Ann. Meet. Japan<br />
Soc. Fert. Implant. 206, 2006)<br />
Figure 3. Development of human embryos cultured in global® from Day 1 to Day 3<br />
and Day 3 to Day 5, or in G1 from Day 1 to Day 3 and then in G2 from Day 3 to<br />
Day 5. The proportion of embryos reaching the blastocyst state by Day 5 was<br />
significantly great in global®. The pregnancy rates were not different between the<br />
culture media treatments. (Angus et al., Fert. Steril. 86 Suppl 2, S229, 2006)<br />
Figure 4. Development of human embryos cultured in global® from Day 1 to Day 3<br />
and Day 3 to Day 5, or in ECM from Day 1 to Day 3 and then in Multiblast from<br />
Day 3 to Day 5. The proportion of embryos ³ 6 cells on Day 3, and at the<br />
blastocyst by Day 5, were significantly great in global®. The clinical pregnancy<br />
rate was not different between the culture groups. (Sepulveda et al., 2008)<br />
Figure 5. Development of human embryos cultured in global® from Day 1 to Day 3<br />
and Day 3 to Day 5, or in Cleavage from Day 1 to Day 3 and then in Blastocyst<br />
from Day 3 to Day 5. The proportion of embryos > 6 cells on Day 3, and at the<br />
blastocyst by Day 5-6, were significantly greater in global®. The clinical pregnancy<br />
rate was not different between the culture groups. (Carrilo & Yalcinkaya, 2008)<br />
• ISO 13485:2003 & 9001:2000 Certified<br />
• Fresh Delivery<br />
• Lot-to-lot consistent results<br />
www.LifeGlobal.<strong>com</strong><br />
US/Canada: 1-800-720-6375 • International: 1-519-826-5800<br />
Fax: 1-519-826-6947 • email: sales@LifeGlobal.<strong>com</strong><br />
www.IVFonline.<strong>com</strong>
Protect Embryos<br />
…Embryos do not have an “immune system”<br />
• VOCs can have significant detrimental effects on embryos.<br />
• VOCs are too small to be trapped by HEPA filters.<br />
• Coda ® filters are <strong>com</strong>mon-sense safety devices that serve to protect embryos from<br />
unforeseen Volatile Organic Contaminants (VOCs) and other contaminants.<br />
Common VOCs found inside incubators, such as, Benzene,<br />
Acetone, Ethanol, and Formaldehyde are 100 to 1000 times<br />
smaller than the pore size of the HEPA filter.<br />
Air contaminants present in research and clinical<br />
laboratories interact with specimens, samples,<br />
tissues, media and oils. Studies show that Chemical<br />
Air Contaminants (CACs) and Volatile Organic<br />
Contaminants (VOCs) introduced from many<br />
sources may seriously distort your results. The<br />
Coda ® Air and Gas Filtration System can maximize<br />
the air quality of your incubators and laboratories by<br />
removing up to 99.97% of these contaminants. The<br />
following table shows the filtration power of the<br />
Coda ® System <strong>com</strong>pared to existing HVAC systems<br />
and HEPA filters used with your equipment. HEPA<br />
filters are designed to stop materials down to 0.3<br />
microns.<br />
μg/m 3<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
Outside air<br />
Return air<br />
Hallways<br />
Procedure<br />
Room<br />
Laminar<br />
flow units<br />
Incubators<br />
Benzene<br />
Toluene<br />
Xylenes<br />
Styrene<br />
Volatile Organic Contaminants in Air Outside and Inside an ART Laboratory<br />
(Cohen et al., Human Reprod. <strong>12</strong>, 1742-1749, 1997)<br />
HEPA filters are designed to stop materials down to<br />
0.3 microns. Unfortunately many of the gases found in<br />
IVF labs; benzene, acetone, ethanol, formaldehyde, etc;<br />
are 100 to 1000 times smaller than the pore size of the<br />
HEPA filter. If improving results is your goal, then Coda ®<br />
is the solution. For more information call IVFonline or<br />
visit our website at www.IVFonline.<strong>com</strong>.<br />
FDA 510(k) Cleared ISO13485:2003 ISO9001:2000<br />
Laboratories<br />
Gas Lines<br />
Incubators
ARTICLES<br />
The identification of a toxic substance in<br />
the in vitro fertilization laboratory: the<br />
value of inter-laboratory <strong>com</strong>munication<br />
by Tom Turner, MS ELD (ABB), Director of Embryology<br />
Austin IVF / Texas Fertility Center, Austin, TX, USA<br />
The quick identification and elimination of toxic<br />
substances within the IVF laboratory is critical for the<br />
growth of quality embryos. In spite of having a wellconstructed<br />
lab and using tested products, unknown<br />
substances can enter the culture system from a variety of<br />
sources and are difficult to pinpoint and eliminate. These<br />
toxins can have a negative impact on embryo<br />
development and lower patients' chances for pregnancy.<br />
We have a well-established, successful IVF clinic that<br />
has been in operation since 1984. In January 2007, we<br />
moved our IVF laboratory into a new facility that was<br />
constructed with all of the current knowledge about A/C,<br />
air filtration, construction materials, and laboratory<br />
techniques.<br />
The culture system used in our IVF laboratory<br />
consists of triple gas incubators that are 1-4 years old.<br />
These are maintained properly and checked daily for<br />
temperature, CO2, and O2. The pH of the equilibrated<br />
culture media is checked weekly. Medical grade gases are<br />
used, and the media are of a sequential system from a<br />
well-known, respected <strong>com</strong>pany offering many IVF<br />
products. Oil is purchased from the same <strong>com</strong>pany that<br />
provides the culture media.<br />
Notably, after fertilization, the embryos are cultured<br />
individually in 25 microliter drops over-layered with<br />
11 ml of oil, within 60 mm Petri dishes.<br />
All of the plastic products used in the laboratory are<br />
mouse embryo tested before they are used in the IVF lab.<br />
They are allowed to aerate while out of their package<br />
before they are used.<br />
The first observations of deteriorating quality of<br />
embryo development occurred in sporadic cases in<br />
November. We observed that day-3 embryos appeared<br />
healthy, while most day 5 blastocysts were still<br />
developing, but not sufficiently developed to freeze. Some<br />
of the embryos that appeared healthy, but not sufficiently<br />
developed to freeze on day 5, had degenerated by day 6.<br />
To clarify, degeneration was the death of all or most of the<br />
cells in the blastocyst. Our concern with these<br />
observations led to a review of our techniques of<br />
blastocyst handling, the embryologists involved,<br />
incubators used, and the<br />
aeration time of our<br />
polystyrene dishes. No<br />
cause was identified.<br />
Some improvement in<br />
blast development was seen<br />
in early December, with no<br />
obvious reason. But by mid<br />
December, we began seeing<br />
more degeneration of<br />
blastocysts on day 6. There<br />
was also a decrease in the<br />
TOM<br />
percent of patients who had<br />
TURNER, MS ELS (ABB)<br />
blastocysts left after transfer that were suitable to freeze.<br />
Since we had a laboratory shutdown scheduled for<br />
late December, we examined all of the options for change<br />
and improvement that we could ac<strong>com</strong>plish during the<br />
month-long break.<br />
During the scheduled shutdown, all of the potassium<br />
permanganate, carbon, and HEPA filters in the laboratory<br />
A/C system were changed. A thorough cleaning,<br />
sterilization and calibration of each piece of equipment<br />
that heated or provided an atmosphere was undertaken.<br />
We ran mouse embryo assays (MEA) on each incubator<br />
and on all of the plastics used in the andrology lab and in<br />
the IVF lab. All of the equipment and the supplies passed<br />
the mouse embryo assay. The lab staff reviewed all<br />
protocols and discussed possible causes of the<br />
deterioration in embryo development.<br />
Patient retrievals began in January with high hopes<br />
that the problem had been eliminated. Immediately, there<br />
appeared the same sporadic degeneration of embryos<br />
from day 5 to day 6.<br />
The manufacturer of our culture medium was<br />
contacted to inform them of the problems that we were<br />
seeing. Because we were told that no other lab had<br />
reported problems to them, we assumed the problem was<br />
internal. The <strong>com</strong>pany had supplied our lab with quality<br />
culture medium for many years and we receive a<br />
certificate of analysis with each product, so it was easy to<br />
look internally for our problems.<br />
64 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
At this point, we talked to embryologists in other parts<br />
of the country who were using the same products. We<br />
found that other programs were having similar problems<br />
that they had yet to identify. Some suspected the medium<br />
or protein. These conversations led to a change of our<br />
blastocyst medium to one that was <strong>com</strong>patible with the<br />
other products we were using. When there was no<br />
improvement, we replaced all of our sequential media and<br />
protein with products from the second <strong>com</strong>pany.<br />
Because numerous emails and phone conversations<br />
with the initial <strong>com</strong>pany failed to offer solutions, we again<br />
began networking with other embryologists. At the same<br />
time, the use of EmbryoMail or ivf.net was not used<br />
because we did not want the <strong>com</strong>pany to be criticized by<br />
individuals who had an agenda for another <strong>com</strong>pany.<br />
Continuation of degenerate blastocysts coupled with<br />
poor re-expansion or degeneration of thawed blastocysts,<br />
led us to suspect that our oil might be the problem.<br />
Immediately, we replaced the oil from the original<br />
<strong>com</strong>pany and began using oil from the new <strong>com</strong>pany for<br />
all of our human embryo culture.<br />
We also ran two MEA’s with medium from the new<br />
<strong>com</strong>pany and each type of oil, deciding to grow the mouse<br />
embryos to day 6. This was because the growth from day<br />
5 to day 6 is where we saw the greatest degeneration.<br />
The results were definitive. All two-celled mouse<br />
embryos cultured in the new <strong>com</strong>pany’s medium overlayered<br />
with the new <strong>com</strong>pany’s oil made hatched<br />
blastocysts by day 6. All mouse embryos cultured with the<br />
new <strong>com</strong>pany’s medium over-layered with the original<br />
<strong>com</strong>pany's oil died by day 6.<br />
Meanwhile, no human blastocysts showed signs of<br />
degeneration while grown in the new medium and the<br />
new oil. The original <strong>com</strong>pany was notified of our<br />
findings.<br />
The identification of the toxic oil that was present in<br />
our lab took much time and effort by the embryologists<br />
and at the expense of human blastocysts. The method to<br />
identify it was painfully slow.<br />
What we learned was;<br />
1. Other labs using the same oil had a similar experience.<br />
2. Labs using the same oil that did not have the same<br />
experience were washing their oil or were growing<br />
embryos in 4-wells with larger volumes of medium<br />
and smaller volumes of oil overlay.<br />
3. Finally, we learned the importance of <strong>com</strong>municating<br />
with other IVF labs and the need for an electronic<br />
forum for discussion of similar problems without<br />
worrying about the expression of <strong>com</strong>mercial<br />
agendas.<br />
You can contact Tom Turner at: tom@austinivf.<strong>com</strong><br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
65
ARTICLES<br />
Clinical Implementation of the Halosperm ® Test Kit in<br />
Combination with SCA ®<br />
by Kellie Williams, PhD and J. Kevin Thibodeaux, PhD, HCLD<br />
Tulsa Fertility Center, 115 East 15th St., Tulsa, OK 74119<br />
Introduction<br />
Efforts to further evaluate the sub-fertile male beyond<br />
less objective parameters such as sperm count, motility<br />
and morphology, have focused on the estimation of sperm<br />
DNA damage. Chromatin within the spermatozoa consists<br />
of DNA, RNA and nuclear proteins. These<br />
macromolecules undergo various changes to achieve<br />
different levels of <strong>com</strong>paction corresponding to the stages<br />
of spermatogenesis and spermiogenesis. The <strong>com</strong>plexity<br />
of sperm chromatin lends itself to an eminent potential for<br />
abnormal chromatin formation and damage to DNA<br />
and/or nuclear proteins. Sperm DNA fragmentation may<br />
occur as a result of six main mechanisms: (1) apoptosis<br />
during spermatogenesis; (2) strand breaks during<br />
chromatin remodeling stages of spermiogenesis;<br />
(3) fragmentation produced by oxygen radicals present in<br />
the seminiferous tubules and the epididymis;<br />
(4) fragmentation induced by sperm caspases and<br />
endonucleases; (5) damage resulting from chemotherapy<br />
and radiotherapy; and (6) damage induced by<br />
environmental toxicants (Sakkas and Alvarez, 2010).<br />
Normal morphology, count and motility may not<br />
necessarily indicate the genomic quality of a fertilizing<br />
sperm. Many mechanisms function to allow only those<br />
sperm with no genetic aberrations to fertilize an oocyte<br />
and initiate embryonic development. Assisted<br />
reproductive technologies, including in-vitro fertilization<br />
(IVF) and intracytoplasmic sperm injection (ICSI), bypass<br />
these checkpoints, and sperm containing chromatin<br />
structure anomalies may be introduced. Research over the<br />
past fifteen years demonstrates a link between damaged<br />
sperm DNA and male sub-fertility, and establishes the<br />
following statistical categories: ≤15% DNA fragmentation<br />
index (DFI) for excellent fertility; >15% to
ARTICLES<br />
sperm with low levels of DNA fragmentation release DNA<br />
loops in an area surrounding the sperm head forming<br />
large halos (Fernandez et al., 2005a). The SCA ® software is<br />
designed to generate DNA fragmentation data from a<br />
specified particle area of stained halos within a captured<br />
image. The diagnostic power of the DNA fragmentation<br />
test is derived from classification of patient test results into<br />
three statistical categories described above (Evenson et al.,<br />
1999).<br />
Our initial control sample was prepared on 11/2008<br />
and was analyzed in parallel on each test day and it<br />
consistently demonstrated DNA fragmentation<br />
repeatability as illustrated in Figure 1. The mean of the<br />
control sample was 50.8% with a coefficient of variation<br />
(CV) of 5.1%. Of the 204 individual patients sampled, the<br />
range of DNA fragmentation was 2 to 100% (Figure 2). The<br />
distribution of patient test values into the three categories<br />
showed 20.1% of patients analyzed with ≤15% DFI<br />
indicating excellent fertility, 22.6% with >15% to
ARTICLES<br />
CONTINUED FROM PAGE 67<br />
References<br />
Evenson DP, Josk LK, Marshall D, Zinaman MJ, Clegg E, Purvis<br />
K, de Angelis P, Claussen OP. (1999) Utility of the sperm<br />
chromatin structure assay (SCSA) as a diagnostic and<br />
prognostic tool in the human fertility clinic. Hum Reprod<br />
14:1039-1049.<br />
Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R,<br />
Alvarez JG. (2003) The sperm chromatin dispersion test: a<br />
simple method for the determination of sperm DNA<br />
fragmentation. J Androl 24:59-66.<br />
Fernandez JL, Muriel L, Goyanes V, Segrelles E, Gosalvez J,<br />
Encisco M, LaFromboise M, De Jonge C. (2005a) Simple<br />
determination of human sperm DNA fragmentation with an<br />
improved sperm chromatin dispersion test. Fertil Steril<br />
84:833-842.<br />
Fernandez JL, Muriel L, Goyanes V, Segrelles E, Gosalvez J,<br />
Enciso M, LaFromboise M, De Jonge C. (2005b) Halosperm<br />
is an easy, available, and cost-effective alternatie for<br />
determining sperm DNA fragmentation. Fertil Steril 84:860.<br />
Sakkas D, Alvarez JG. (2010) Sperm DNA fragmentation:<br />
mechanisms of origin, impact on reproductive out<strong>com</strong>e, and<br />
analysis. Fertil Steril 93:1027-1036.<br />
Schlegel PN, Paduch DA. (2005) Yet another test of sperm<br />
chromatin structure. Fertil Steril 84:854-859.<br />
You can contact Kellie Williams at: kwilliams@obgynmail.<strong>com</strong><br />
Figure 2: Individual patient results for DNA fragmentation testing.<br />
100<br />
90<br />
80<br />
Percent Fragmented DNA<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
1<br />
611162<strong>12</strong>63136414651566166717681869196<br />
101<br />
106111<br />
116<strong>12</strong>1<strong>12</strong>6131136<br />
141146<br />
151156161166171<br />
176181186191196<br />
201<br />
Sequential Patient Results<br />
Figure 3: Distribution of patient test results.<br />
100<br />
90<br />
80<br />
70<br />
Pecent of Patients<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
30%<br />
Percent Fragmented DNA<br />
68 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
ARTICLES<br />
A Prospective Randomized Comparison of global ® Medium with Sequential Media<br />
for Culture of Human Embryos to the Blastocyst Stage<br />
In a recent report, Sepulveda et al. (2009) <strong>com</strong>pared Global medium with ECM/Multiblast<br />
sequential media for the culture of human embryos from the zygote to the blastocyst stage.<br />
Oocytes were collected from 47 oocyte donors and fertilized with sperm of the partners of<br />
80 patients who had been randomly assigned to have their embryos cultured in either<br />
Global (N = 287 zygotes) or ECM/Multiblast (N = 322 zygotes). The zygotes were cultured<br />
from Day 1 to Day 3 in droplets of Global and then in fresh droplets of Global from Day 3<br />
to Day 5 or 6, or in droplets of ECM from Day 1 to 3 and then in droplets of Multiblast from<br />
Day 3 to Day 5 or 6.<br />
On Day 2, cell number was significantly greater (P = 0.022) and multinucleation was<br />
DON<br />
significantly less (P = 0.020) for embryos cultured in Global than for those cultured in<br />
RIEGER, PHD<br />
ECM/Multiblast. As shown in Figure 1, the proportion of zygotes that developed to ≥ 6 cells on Day 3, <strong>com</strong>pacted on Day<br />
3, morula on Day 4, blastocyst on Day 5 and full to hatching blastocyst on Day 5 were significantly greater in Global than<br />
in ECM/Multiblast. There were no other significant differences in developmental parameters between the culture medium<br />
treatments.<br />
On Day 5 or 6, the best (usually two) embryos in each cohort were transferred to 40 patients in the Global group and to<br />
38 patients in the ECM/Multiblast group (mean of 2.0 embryos/transfer in both groups). As shown in Figure 2, there were<br />
no significant differences in pregnancy rates, as measured by being positive for hCG or for fetal heart beats (FHB),<br />
between the culture treatments. However, the implantation rate, as measured by FHB, was significantly greater for<br />
embryos cultured in Global than for those cultured in ECM/Multiblast.<br />
The authors conclude that “A single medium was as good as or better than a sequential media system for human embryo<br />
culture from the zygote to blastocyst stage.” and that “The idea that a sequential media system is required for optimal<br />
development of the human embryo to the blastocyst stage is highly questionable.”<br />
Figure 1: Percent of zygotes developing to the given stages on Days<br />
3, 4, and 5 of culture<br />
Figure 2: Pregnancy and implantation rates<br />
Reference: Sepulveda S, Garcia J, Arriaga E, Noriega PL, Noriega HL (2009) In vitro development and pregnancy<br />
out<strong>com</strong>es for human embryos cultured in either a single medium or in a sequential media system. Fertil<br />
Steril 91, 1765-70.<br />
(Text and figures by D. Rieger, LifeGlobal LLC)<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
69
I’ve planned for this my entire life.<br />
Mr. Right…<br />
the wedding…<br />
the house…<br />
the children…<br />
But I never planned on<br />
possible infertility.<br />
Luckily, I found FertileAge®<br />
fertility supplements for<br />
Women and Men to help me<br />
and my husband conceive.<br />
Order Online at www.FertileAge.<strong>com</strong><br />
FDA Statement<br />
† These statements have not been evaluated by the Food and Drug Administration. These products are not intended<br />
to diagnose, treat, cure, or prevent any disease.<br />
www.FertileAge.<strong>com</strong><br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
email: sales@FertileAge.<strong>com</strong>
GPS Dishware TM<br />
…the future of ART and other sensitive culture<br />
Specific dishes designed, manufactured, tested and certified for ART procedures.<br />
Universal GPS ®<br />
Our new Universal GPS ® dish gives you:<br />
The 8 outer wells will each hold up to 100 μl of medium, and the 2<br />
inner wells will each hold up to 150 μl.<br />
embryo GPS ®<br />
The embryo GPS ® gives major advantages when culturing.<br />
• Each dish has structured wells eliminating collapsing droplets.<br />
• Each well holds a defined volume of media to reduce set up time.<br />
• The bottom of each well has our unique GPS feature – the embryos<br />
are at the center of the well, and at the same focal distance.<br />
The 3 large center wells and 8 outer wells give you consistency. Large<br />
wells for flexibility in single or group culture. The 8 outer wells will<br />
each hold up to 50 μl of medium and the 3 inner wells will each hold<br />
up to 100 μl. Embryo culture has be<strong>com</strong>e simpler and safer.<br />
• Larger wells than the embryo GPS ® for the use of larger volumes<br />
of medium.<br />
• Each dish has structured wells eliminating collapsing droplets<br />
and increasing safety.<br />
• The bottom of each well has our unique GPS feature – the<br />
embryos are at the center of the well, and at the same focal<br />
distance.<br />
embryo corral ®<br />
The embryo corral ® gives you:<br />
• An innovative design that gives you the advantages of group<br />
culture, while maintaining the ability to monitor the<br />
development of each embryo individually.<br />
• The dish allows media and autocrine and paracrine growth<br />
factors to move among the quadrants and thereby shared among<br />
the embryos.<br />
• The bottom of each well and each quadrant of the corral ® well<br />
has our unique GPS feature – the embryos are at the center of the<br />
well, and at the same focal distance.<br />
• Each dish has structured wells eliminating collapsing droplets.<br />
The 2 large inner wells will each hold up to <strong>12</strong>0 μl (30 μl/quadrant) of medium and allow the flexibility in<br />
single or group culture. The 8 outer wells each will hold up to 50 μl of medium, and can be used for culturing,<br />
washing or preparation.<br />
SunIVF<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
email: gps@IVFonline.<strong>com</strong><br />
www.SunIVF.<strong>com</strong><br />
www.IVFonline.<strong>com</strong>
PATIENT’S CORNER<br />
“Morning Sickness” – An overview<br />
by Michele Brown, MD, FACOG<br />
Michele Brown, M.D. FACOG A practicing obstetrician of 26 years and a founder of Beaute<br />
de Maman, a line of personal care products for pregnant women.<br />
You can contact Michele Brown, MD at: mbrownmd54@gmail.<strong>com</strong><br />
Nausea and vomiting often referred to as ‘morning<br />
sickness’ are <strong>com</strong>mon and extremely distressing<br />
symptoms of pregnancy. The term “morning<br />
sickness” is a misnomer as most women suffer from theses<br />
symptoms throughout the day.<br />
Women often experience some nausea in the early<br />
stages of pregnancy. In most cases the symptoms are mild<br />
transient and easily managed by dietary modifications. In<br />
some cases however, the symptoms may be severe.<br />
Relentless vomiting can last the entire pregnancy and be<br />
associated with dehydration and weight loss.<br />
Even in the milder cases nausea and vomiting can<br />
affect the pregnant woman’s outlook on the pregnancy,<br />
lead to significant distress, and interfere with the<br />
nutritional requirements.<br />
Some important facts about nausea and vomiting in<br />
pregnancy<br />
• Incidence: Nausea and vomiting of pregnancy are<br />
extremely <strong>com</strong>mon and occur in 50 to 80% of pregnant<br />
women.<br />
• Time of occurrence: In most cases symptoms usually<br />
appear by the 5th week and disappear by the 13th<br />
week of pregnancy. Severity peaks between 11 and 13<br />
weeks. However, in 20% of women, nausea and<br />
vomiting can persist throughout pregnancy.<br />
• The Causes of nausea and vomiting in pregnancy are<br />
largely unknown. However, the following<br />
associations have been noted.<br />
1. Conditions in which pregnancy hormone levels<br />
are high (twin pregnancy, molar pregnancy) are<br />
associated with a higher incidence of nausea and<br />
vomiting. On the other hand, women whose<br />
pregnancy hormones are low (smokers) have a<br />
lower incidence of nausea and vomiting.<br />
2. Women taking prenatal vitamins are less likely to<br />
have severe nausea and vomiting.<br />
3. Psychological causes and transformation of a<br />
mental disorder into physical symptoms<br />
(psychosomatic conditions) have little supporting<br />
evidence as the cause of nausea and vomiting.<br />
However, nausea and vomiting especially in their<br />
severe forms can cause severe psychological<br />
MICHELE BROWN, MD, FACOG<br />
distress, which in turn may worsen the condition.<br />
4. Genetic susceptibility: A history of hyperemesis is<br />
found in other family members suggesting a<br />
genetic link.<br />
5. History of nausea and vomiting in a previous<br />
pregnancy suggesting individual susceptibility.<br />
6. History of migraine headaches is linked to nausea<br />
and vomiting.<br />
7. Women on their first pregnancy are less likely to<br />
develop hyperemesis as <strong>com</strong>pared to subsequent<br />
pregnancies.<br />
8. Nausea and vomiting are more <strong>com</strong>monly found<br />
when the interval between pregnancies is short.<br />
Hyperemesis Gravidarum:<br />
An extreme form of this <strong>com</strong>mon symptom. In 1 to 3%<br />
nausea and vomiting are severe leading to dehydration<br />
and electrolyte imbalance and weight loss occasionally<br />
requiring hospitalization.<br />
In addition to the severe psychological impact of<br />
hyperemesis, it can also lead to serious pregnancy<br />
<strong>com</strong>plications or death;<br />
• Rupture of the esophagus and bleeding from ruptured<br />
blood vessels.<br />
• Wernicke’s encephalopathy-a rare but serious brain<br />
disorder associated with Vitamin B1 deficiency and<br />
leading to memory loss, visual disturbances, and gait<br />
disturbances.<br />
• Low birth weight as a result of malnutrition.<br />
• Pregnancy termination due to severe psychological<br />
distress and depression.<br />
CONTINUED ON PAGE 74<br />
72 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
PATIENT’S CORNER<br />
CONTINUED FROM PAGE 72<br />
Treatment of nausea and vomiting in pregnancy:<br />
In most cases the purpose of the treatment is to<br />
improve the pregnant woman’s quality of life. In cases of<br />
hyperemesis treatment is essential and can be lifesaving.<br />
No single specific treatment is effective in all women.<br />
A carefully tailored multidisciplinary approach which<br />
includes dietary modifications, herbal remedies and<br />
medication should be adjusted to the woman's needs.<br />
Emotional support is extremely valuable.<br />
Dietary Suggestions<br />
• Avoid foods and odors that might trigger the<br />
symptoms.<br />
• Eat bland high protein and carbohydrate foods and<br />
snacks—avoid fatty, spicy foods, short frequent meals<br />
help. Solid starches such as potatoes, rice, and pasta<br />
are re<strong>com</strong>mended.<br />
• Stop all iron tablets.<br />
• Avoid dehydration (1.0 to 1.5 liter) by drinking sports<br />
drinks and bouillon which contain salt, glucose, and<br />
potassium. Ginger ale is another traditional remedy.<br />
Advance to brothy soups with noodles or rice. Avoid<br />
cream based soups because of fat content.<br />
are severely dehydrated. In addition to proper<br />
hydration, intravenous medications are used (Reglan,<br />
Phenergan, Dramamine, and Zofran).<br />
Tigan suppositories can also be of value in women<br />
who cannot tolerate oral medications.<br />
Steroid therapy has been reported to be successful in<br />
some resistant cases but should be avoided if possible<br />
due to the associated risk of fetal malformation (cleft<br />
palate) when given within the first 10 weeks of<br />
pregnancy.<br />
• Vitamin B1 therapy: All women who have been<br />
vomiting for 3 weeks and require IV hydration should<br />
be given supplemental vitamin B1 to prevent<br />
Wernicke's encephalopathy.<br />
In summary: Nausea and vomiting of pregnancy is<br />
extremely <strong>com</strong>mon and a normal part of a healthy<br />
pregnancy in most cases. In severe cases, hospitalization<br />
may be necessary along with lost time from work, and<br />
multiple visits to your obstetrician. There are multiple<br />
theories and mechanisms that can cause this problem.<br />
Herbal and natural remedies<br />
• Vitamin B6 (pyridoxine 10-25 mg taken 3 or 4 times a<br />
day) is used due to its anti-emetic properties. An<br />
antihistamine (doxylamine sold as over the counter as<br />
Unisom sleep tabs 1/2 tab) can be added to the<br />
pyridoxine as first line therapy (10 mg of each).<br />
• Ginger tablets (250 mg given 3 or 4 times a day or<br />
powdered ginger extract 1 gram/day).<br />
• Acupuncture, acupressure (wristbands), and<br />
hypnosis.<br />
Drug Therapy<br />
In most cases the condition can be managed on an<br />
outpatient basis with close follow up by the obstetrician to<br />
ensure proper hydration and nutrition.<br />
• Drugs that are administered on an outpatient basis fall<br />
into the following categories 1. Antiemetic drugs. e.g.<br />
Zofran, Tigan, Reglan, 2. Phenothiazines e.g.<br />
Promethazine, 3. Anti-histamines and 4. Steroids.<br />
• IV fluid therapy, intravenous nutrition and<br />
hospitalization may be required for those women who<br />
74 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
Vitamin D Deficiency and Pregnancy<br />
by Michele Brown, MD, FACOG<br />
PATIENT’S CORNER<br />
What is Vitamin D<br />
Vitamin D is a fat-soluble vitamin that plays a central<br />
role in calcium and phosphorous metabolism, which is<br />
critical for bone formation and maintenance.<br />
Why is Vitamin D important<br />
Years ago, deficiency of this vitamin resulted in<br />
rickets. New studies have demonstrated a resurfacing of<br />
Vitamin D deficiency worldwide. This deficiency is<br />
especially critical in the pregnant and lactating woman.<br />
Studies have shown how important Vitamin D is to<br />
skeletal, cardiovascular, and neurological development in<br />
the infant. Other studies have shown Vitamin D deficiency<br />
to be linked to diabetes, asthma, and schizophrenia in<br />
children. Infants born to mothers with Vitamin D<br />
deficiency had poor growth, and defects in enamel<br />
formation of teeth. A newborn’s Vitamin D level is<br />
<strong>com</strong>pletely dependent on maternal levels.<br />
Vitamin D is believed to be critical in placental<br />
development and function, which may be associated with<br />
other <strong>com</strong>plications during pregnancy including<br />
miscarriage, preeclampsia, and preterm birth. Low<br />
Vitamin D levels have been linked to bacterial infections in<br />
the vagina in the first trimester of pregnancy. This can<br />
increase the risk of preterm birth and adverse pregnancy<br />
out<strong>com</strong>es. One study found a four-fold greater risk of<br />
cesarean section for women with low Vitamin D levels.<br />
This may be due to the fact that skeletal muscle contains<br />
Vitamin D receptors and deficiency can result in muscle<br />
weakness and poor strength in labor. Vitamin D also<br />
regulates calcium levels and, with deficiency, muscle<br />
strength is lowered in labor.<br />
Where do we get Vitamin D<br />
Exposure to sunlight – An inactive form of Vitamin D<br />
is synthesized in the skin by exposure to ultraviolet light,<br />
and the inactive form is converted to the active form by the<br />
liver and the kidney.<br />
Diet – Very few food sources naturally contain<br />
vitamin D. Examples are oily fish such as salmon,<br />
sardines, mackerel, and tuna along with egg yolks, and<br />
fish liver oils. Foods such as milk, orange juice, some<br />
cereals, yogurt, cheese, and butter are often fortified with<br />
Vitamin D.<br />
Supplements – Over-the-counter supplements are<br />
precursors to Vitamin D, which get converted in the body.<br />
What is considered to be a Vitamin D deficiency<br />
Deficiency is defined as serum levels of less than 20<br />
mg/ml of 25-hydroxy Vitamin D. Levels between 20 and<br />
30 mg/ml indicate insufficiency, and anything above 30<br />
mg/ml is considered normal. Toxic levels are over 150<br />
mg;/ml and are exceedingly rare.<br />
Deficiencies appear to be more <strong>com</strong>mon among<br />
African-Americans, due to high levels of melanin which<br />
blocks light from entering the skin. In addition, levels are<br />
lower in the winter months (November through March)<br />
when less sunlight reaches the earth. Levels are low in<br />
people living above 30 degrees latitude, in cultures where<br />
the skin is covered (e.g. Arab countries), and in cultures<br />
where people avoid sunlight and use sunscreen.<br />
Certain medical conditions make one more prone to<br />
deficiency such as individuals with bowel absorption<br />
problems, people with renal disease, obese individuals,<br />
vegetarians, people with lactose intolerance, and people<br />
on certain medications, including anticonvulsants.<br />
What are the requirements for vitamin D in pregnant<br />
and lactating women<br />
Current research has shown that pregnant women are<br />
at high risk of Vitamin D insufficiency and prenatal<br />
vitamins are inadequate to meet these demands. Current<br />
vitamin preparations have approximately 200 to 400 IU of<br />
Vitamin D. Experts re<strong>com</strong>mend 1400 to 2000 IU of Vitamin<br />
D per day during pregnancy. This can be ac<strong>com</strong>plished<br />
with regular prenatal vitamins in addition to another<br />
supplement. It has been suggested that breastfeeding<br />
women, whose infants only get vitamin D from breast<br />
milk, need to ingest 4000 to 6000 IU of Vitamin D per day.<br />
Serum Vitamin D levels should be determined at the<br />
first prenatal visit along with the other prenatal blood<br />
work. If women are Vitamin D deficient, they should be<br />
treated with 2000 IU of vitamin D in addition to their<br />
prenatal vitamins for 1-2 months and the blood test<br />
repeated to confirm that the serum level is above<br />
30 mg/ml. With continued supplementation of 1000 IU of<br />
Vitamin D per day, levels should be sufficient for the<br />
remainder of the pregnancy. Borderline individuals might<br />
need to be tested again during the third trimester.<br />
Ultraviolet light exposure can also increase the<br />
vitamin D content of human milk.<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
75
PATIENT’S CORNER<br />
Too Much of a Good Thing<br />
by Courtney Sirotin<br />
Atlanta, GA, USA<br />
Have you ever wished your period would just<br />
magically go away For some women in their<br />
child-bearing years, it does. As good as that may<br />
sound on the days that you’re doubled over in pain with a<br />
heating pad, losing your period is a lonely, terrifying<br />
experience with repercussions ranging from osteoporosis<br />
to infertility. I speak from experience because it happened<br />
to me.<br />
My husband and I waited awhile before trying to have<br />
a baby. I was thirty years old when he finally said the<br />
magic words, “I think I'm ready.” I was overjoyed and<br />
went off my birth control pills immediately. I had heard<br />
that a woman is extra fertile the month after going off The<br />
Pill, so I quickly adapted the habits of a pregnant woman,<br />
popping prenatal vitamins and giving up caffeine, in<br />
anticipation of a positive pregnancy test. When I didn’t get<br />
pregnant that next month, and when I didn’t have a<br />
period, I chalked it up to having just gone off the birth<br />
control pills that I had been taking for seven years. I had<br />
read that some women have irregular cycles for awhile<br />
after going off the pill, so I wasn't overly concerned.<br />
For the next few months, I continued to live my life as<br />
usual. In the previous two years, my husband and I had<br />
moved to a new state, and I stayed at home to pursue a<br />
career in writing. I had a lot of time on my hands and<br />
without even being conscious of it I had taken what was<br />
once a healthy habit, and turned it into an unhealthy<br />
extreme. I’m talking about exercise.<br />
I've always worked out, but never more than an hour<br />
a day. When we moved and my free time increased, I<br />
naturally started filling it up with more activity. At first I<br />
just added strength training to my regimen, something I<br />
had done before but only half-heartedly. Then I got a<br />
subscription to a fitness magazine and learned about<br />
things like intervals and plyometrics. Every time I learned<br />
something new about fitness, a new move or a new style<br />
of exercise, I just added it onto what I was already doing<br />
each day, rather than spreading things out over the course<br />
of a week or a month. It got to the point where I was<br />
working out hard for hours and hours each day and rarely,<br />
if ever, giving myself a day off to recover.<br />
Some days I would wake up and go for a seven mile<br />
run, <strong>com</strong>e home and lift weights for an hour or two,<br />
shower, eat a bowl of cottage cheese with strawberries,<br />
and then do some writing until my husband came home<br />
from work, at which time we would work out together. At<br />
night I might go for another run and do intervals, maybe<br />
COURTNEY SIROTIN<br />
plyometrics, maybe lift more weights, maybe all three. The<br />
more I worked out, the more obsessed I became. I created<br />
a website and blogged about fitness and even wrote a<br />
book on the topic. At that point I had also be<strong>com</strong>e a fitness<br />
trainer and spent the few hours I was not working out on<br />
my own, working out along side my clients, at times<br />
pushing them to do more than they were ready for<br />
because I wanted to go faster or work harder alongside<br />
them.<br />
I never consciously restricted my eating. I’ve always<br />
eaten two thousand calories or more each day, but even<br />
three thousand or four thousand may not have been<br />
enough to fuel my body when I was at my extreme.<br />
Needless to say I was thin. This was a reward<br />
considering I have never been svelte by nature. Growing<br />
up I was a thick, chunky child and remained that way<br />
most of my teenage years. Working out had been my trick<br />
to staying at a healthy weight for many years, that is, until<br />
my working out got out of control. As thin as I was, I<br />
didn't take much time to appreciate my size zero jeans<br />
because I was always in my workout clothes getting<br />
sweaty. I was thin, hungry, tired, and not happy.<br />
So why was I so confused when I didn’t get my period<br />
back after going off The Pill It doesn’t make sense, does<br />
it I should have realized it was because of my lifestyle.<br />
But that’s not the way it works when you are in the throes<br />
of something so extreme. Even my husband, who had<br />
been by my side through the whole process, never<br />
suspected my extreme exercising was having an effect on<br />
my menstrual cycle. But in the dark, in that place where a<br />
woman just knows, deep down, I knew.<br />
And because I knew somewhere deep inside me, I<br />
used the right keywords when I finally investigated my<br />
missing periods on Google: ‘extreme exercise,’ ‘low-fat<br />
diets,’ and ‘amenorrhea.’ That’s how I came across a<br />
message board for women suffering from a condition<br />
78 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
PATIENT’S CORNER<br />
called hypothalamic amenorrhea. I read the first post and<br />
immediately identified with the writer.<br />
According to the Mayo Clinic, the definition of<br />
hypothalamic amenorrhea (HA), or secondary<br />
amenorrhea, is the absence of menstruation in women<br />
who previously had regular periods. The term<br />
hypothalamic refers to the hypothalamus, an area at the<br />
base of the brain that acts as a hormone control center for<br />
the body, regulating, among other things, a woman’s<br />
menstrual cycle. In certain situations, such as anorexia,<br />
excessive exercise and stress, the flow of hormones is<br />
interrupted. This results in the failure of the body to<br />
produce enough estrogen and progesterone, the<br />
suppression of ovulation, and, ultimately, the loss of<br />
menses.<br />
Excessive exercise Check. Anorexia In a way,<br />
because I wasn’t eating enough calories to sustain my<br />
activity level. Check. Stress You think it’s relaxing to do<br />
high-intensity interval training on a daily basis No, it’s<br />
stressful! Check. I had the trifecta.<br />
There were thousands of entries on that message<br />
board and I read every last one. When I had finished,<br />
about a week later, I knew everything there was to know<br />
on the topic. What I learned was that there was only one<br />
way for me to ever have a chance of having a baby: I had<br />
to stop exercising, eat fat, and gain weight. It’s an<br />
evolutionary thing. Back in the days of early humanity<br />
there were times of feasts and times of famine. There were<br />
times when a woman would be running from predators<br />
and times she would be nesting and taking care of her<br />
family. While I was exercising like my life depended on it,<br />
my body was registering the fact that I was living in a time<br />
of stress and famine, when it is not prudent to bring a child<br />
into the world. As a result, my body was shutting down<br />
my reproductive system in order to prevent me from<br />
getting pregnant. The only way I could reverse the<br />
damage was to convince my body the famine was over and<br />
times were looking plentiful again.<br />
My gynecologist confirmed my self-diagnosis through<br />
simple blood work that revealed my estrogen, luteinizing<br />
hormone, and follicle stimulating hormone levels were<br />
basically nonexistent. She encouraged me to take the next<br />
year to forget about getting pregnant and just focus on<br />
getting my body to a safe place. Because of the risk of low<br />
bone density surrounding low estrogen levels, I was also<br />
instructed to have a bone density test done and,<br />
thankfully, it came back normal.<br />
I have been ready to be a mother for as long as I can<br />
remember. In fact, I think the intensity of my exercise<br />
regimen was, in part, a way to numb the desire I was<br />
having, as I was entering my thirties, to have a baby. I was<br />
filling up the days and nights of what should have been<br />
my motherhood with a distraction. Facing the fact that I<br />
was infertile was devastating, but once I had that<br />
information in hand, I didn’t think twice about doing<br />
everything in my power to reverse the damage. I stopped<br />
working out immediately and started eating more than my<br />
activity level required. This was by no means easy, as<br />
fitness had be<strong>com</strong>e not only my obsession but my identity,<br />
but I just kept reminding myself that I wanted to have a<br />
baby more than I wanted to be thin.<br />
A year later I went to see a reproductive<br />
endocrinologist, a specialist in infertility. I had done<br />
everything right: I gained twenty pounds, ate plentifully,<br />
and the only activity I engaged in was light, gentle<br />
walking. I had not resumed menstrual periods on my own<br />
yet, but I felt healed. I wanted to know what more I could<br />
do to bring a life into this world.<br />
My blood work came back with wonderful news. All<br />
of my hormones were back in normal ranges. I was<br />
overjoyed but also curious as to why I had not resumed<br />
menstruating. The doctor explained to me that with HA it<br />
can be a long, slow road to recovery and that every woman<br />
is different. Some women resume menstruating quickly,<br />
other take years, some never menstruate again.<br />
Because of my hormone levels, I was a candidate to<br />
take a fertility drug called Clomid. It is one of the most<br />
<strong>com</strong>mon, least expensive, treatments for infertility. Clomid<br />
indirectly stimulates ovulation which may increase your<br />
chance to conceive. I went through four cycles of Clomid<br />
and was about to give up further treatments until I could<br />
afford in-vitro fertilization when I received a positive<br />
pregnancy test. Using Clomid only worked for me because<br />
I was ready. If I had not taken that year to heal, I do not<br />
think I would be pregnant today, twenty pounds heavier<br />
and a million times happier.<br />
Pregnancy has been known to “reset” some woman’s<br />
menstrual cycles and I am hoping it does so for me. I have<br />
learned many things through this experience but mostly<br />
I’ve learned to never let an obsession take over my life<br />
again. If I ever wear a size zero again I'll know I am doing<br />
something very, very wrong. Avoiding extremes is a<br />
challenge for someone with my personality type, but my<br />
goal for my own future, and for that of the baby inside me,<br />
is to live a life of moderation in all things and to pass that<br />
value along.<br />
If you are an excessive exerciser and missing your<br />
period, please consider the possibility that you have<br />
hypothalamic amenorrhea and see a gynecologist for a<br />
diagnosis. There are many other reasons that a woman<br />
might stop having her period and all of them are<br />
important to diagnose because having a menstrual cycle<br />
every month is a barometer of a woman’s health.<br />
CONTINUED ON PAGE 80<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
79
PATIENT’S CORNER<br />
CONTINUED FROM PAGE 79<br />
Remember that birth control creates a synthetic menstrual<br />
cycle and can mask amenorrhea, so if you are concerned<br />
you might have it consider stopping the pill temporarily<br />
and using a back up form of birth control until you know<br />
for sure that you are cycling normally. And if you ever<br />
want to have kids, please don't take your womanhood for<br />
granted. Not having hormones is a major drag.<br />
Note from the editor:<br />
As the author notes, excessive exercise can be a cause<br />
of secondary amenorrhea, and thereby loss of fertility.<br />
However, it is extremely important to know that there are<br />
many other causes of secondary amenorrhea, including<br />
physiological disturbances and pharmaceuticals. Many of<br />
these can have serious and long-term effects if not treated<br />
promptly. Medline Plus, a service of the U.S. National<br />
Library of Medicine and the National Institutes of Health,<br />
contains the following advisory (my emphasis):<br />
“Call for an appointment with your primary health care<br />
provider or OB/GYN provider if you are a woman and have<br />
missed more than one period so that the cause, and<br />
appropriate treatment, can be determined.”<br />
(http://www.nlm.nih.gov/medlineplus/ency/article/00<br />
<strong>12</strong>19.htm, accessed May 20, 2010)<br />
You can contact Courtney Sirotin at: courtney.sirotin@gmail.<strong>com</strong><br />
80 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
• Designed for IVF<br />
• Prewashed extensively with ultra-pure water<br />
• Pharmaceutical grade quality mineral oil<br />
• Sterile Filtered (SAL 10–3)<br />
• 1 cell MEA and Endotoxin Tested<br />
• Optimal viscosity for embryo culture<br />
• 24 Month minimum shelf life<br />
• Prewashed extensively with ultra-pure water<br />
• Pharmaceutical grade, light paraffin oil<br />
• Sterile Filtered (SAL 10–3)<br />
• 1 cell MEA and Endotoxin Tested<br />
• 24 Month minimum shelf life<br />
www.LifeGlobal.<strong>com</strong><br />
• Proven in IVF Labs Worldwide<br />
• Maximum Air Flow of 530 Cubic Feet/Minute<br />
• 1 Year Warranty<br />
• Effective Coverage of up to 400 Square Feet<br />
The Coda ® Aero is a technology that is designed to improve the air health conditions in your IVF laboratories<br />
and workspace.<br />
Our Coda ® Technology reduces particulates, harmful volatile organic <strong>com</strong>pounds (VOCs) and chemical<br />
airborne contaminants (CACs).<br />
Our specially designed HEPA filters remove 99.97% of contaminants and is <strong>com</strong>bined with our unique blend<br />
of activated carbon and alumina impregnated with potassium permanganate for optimum absorption and<br />
oxidation of a wide variety of gases and particulate contaminants.<br />
Coda ® is the ‘First’ product in laboratory air purification and has been the most successful for the last 7 years.
Traditional Petri Dish<br />
Embryo GPS ® Dish<br />
→ running droplets<br />
→ droplets mixing<br />
→ droplets flattening<br />
→ poor temperature control<br />
→ losing track of marked droplet<br />
→ difficulty locating embryos<br />
→ sealing of dish lid & increased pH<br />
‘drop-less’ environment<br />
micro wells designed to enhance embryo culture<br />
no droplets collapsing and mixing<br />
save time and money by reducing set up time,<br />
observation time, testing and handling<br />
better heat conservation<br />
enhance safety<br />
easily locate & observe embryos<br />
new designed lid for better gas exchange<br />
standardization of culture, uniform volumes<br />
1-cell MEA and LAL testing by independent<br />
<strong>com</strong>pany<br />
Standardize ART and other sensitive culture by using GPS Dishware TM<br />
Eliminate these concerns and enhance safety by using GPS Dishware TM : embryo GPS ® , embryo<br />
corral ® , and Universal GPS TM .<br />
embryo GPS ® – The best dish for PGD Cases<br />
Introducing a new dish design for larger volume culture.<br />
Patented & Patent Pending<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
email: gps@IVFonline.<strong>com</strong><br />
www.SunIVF.<strong>com</strong><br />
www.IVFonline.<strong>com</strong>
NEW PRODUCTS<br />
Powder Free Surgical Gloves<br />
Pristine gloves are <strong>com</strong>pletely free of<br />
talcs, starches, calcium carbonates or<br />
powdered substances or lubricants of any<br />
kind. Developed to protect the patient<br />
from granuloma, peritonitis, adhesions<br />
and other powder-related <strong>com</strong>plications,<br />
Pristine gloves are also designed to<br />
protect against powder-related dermatitis<br />
and other powder-associated irritations of<br />
the hands and skin.<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
sales@IVFonline.<strong>com</strong><br />
www.IVFonline.<strong>com</strong><br />
84 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
NEW PRODUCTS<br />
LifeGlobal® Protein Supplement<br />
MatriPlus TM liver biomatrix and our<br />
StemQ® Media to grow differentiate<br />
and maintain hepatocytes…<br />
StemQ® HepatoGro TM<br />
StemQ® HepatoDiff TM<br />
StemQ® HepatoMainDiff TM<br />
LifeGlobal® Protein Supplement with<br />
the enhanced performance provided by<br />
α- & β-globulins.<br />
• Tested lot-to-lot<br />
• Stringent quality control and testing<br />
• One-cell Mouse Embryo Assay Testing<br />
• <strong>12</strong>-month shelf life<br />
• pH, Osmolality and Endotoxin Tested<br />
• High quality of manufacturing<br />
• ISO 13485:2003 & 9001:2000 Certified<br />
StemQ® Scalable Human Neural<br />
Model System<br />
An In Vitro Regenerative Model<br />
global® Blastocyst Vitrification System<br />
Based on S 3<br />
DMSO-free and regular sealable straws<br />
global® Blastocyst Vitrification Kit<br />
– Based on S3<br />
High quality lite mineral<br />
oil is sterile-filtered and<br />
QC tested. MEA and<br />
endotoxin tested.<br />
• Novel serum-free media formulations<br />
– StemQ® NeuroGro Growth Media<br />
– StemQ® NeuroDiff Differentiation Media<br />
• Human Neural Progenitor Cells (NPCs)<br />
– Isolated from human brain tissue<br />
– 11 Donor Lots available (genetic diversity)<br />
– Low passage number<br />
– Average doubling time of 45 hours<br />
– Differentiates into multiple neuronal subtypes,<br />
astrocytes and oligodendrocytes<br />
– Scalable (109 cells)<br />
– Consistent Reproducible Results (single batch)<br />
• Can be used in a High Throughput Screening<br />
Platform<br />
global® Blastocyst Vitrification Thawing Kit<br />
– Based on S3<br />
Paraffin Oil is<br />
sterile-filtered and<br />
QC tested. MEA<br />
and endotoxin<br />
tested.<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
85
BOOKS<br />
DEBRAH FRANK, CH, CI<br />
About the Author<br />
Debrah Frank has been<br />
writing short stories, poetry<br />
and articles for most of her<br />
life. She is a Reiki Master,<br />
Certified Reflexologist and a<br />
Certified Hypnotherapist as<br />
well as instructor and has<br />
been working in the field of<br />
alternative healing for quite<br />
some time.<br />
You can be, do and have anything imaginable. All you<br />
need is a little bit of self awareness. Awareness is the<br />
starting point of all personal growth. Once you be<strong>com</strong>e<br />
aware of something it is then that you can change it.<br />
Without awareness nothing changes.<br />
Your Personalized copy of “Inner Metamorphosis”<br />
can be purchased at: www.PersonalDynamix.<strong>com</strong><br />
About the Author<br />
Harry Fisch, MD, Columbia<br />
University Medical Center, NY,<br />
author of “The Male Biological<br />
Clock”, is one of the nation’s<br />
leaders in the diagnosis and<br />
treatment of male infertility.<br />
HARRY FISCH, MD<br />
The Male Biological Clock can be purchased at:<br />
www.malebiologicalclock.<strong>com</strong><br />
86 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
RESOURCES<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
87
RESOURCES<br />
The New England Fertility Institute is a patient oriented, full service treatment<br />
center, providing state-of-the-art, caring, fertility services.<br />
Dr. Gad Lavy and his trained team of fertility specialists are here to help you, by<br />
treating both female and male infertility with the most up-to-date techniques, in<br />
a modern facility.<br />
The New England Fertility Institute is consistently ranked among the top fertility<br />
clinics in the United States for the past 15 years. Come share in our success.<br />
New England Fertility Institute<br />
<strong>12</strong>75 Summer Street, Stamford, CT, USA 06905 • 203-325-3200<br />
9 Washington Avenue, Hamden, CT, USA 06518 • 203-248-2353<br />
Visit our website at www.nefertility.<strong>com</strong> to see our success. Contact Dr. Lavy at<br />
glavy@nefertility.<strong>com</strong>. We are conviently located close to New York City and major airports.<br />
88 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
RESOURCES<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
89
RESOURCES<br />
Our Experts:<br />
Megan Freebury Karnis, BA/Sc, MD, FRCSC<br />
Shilpa Amin, BSc, MD, FRCSC<br />
Michael Neal, BSc (Hons), MSc, PhDc<br />
Edward G. Hughes, MBChB, MSc, FRCSC<br />
Caroline Beliveau, MD, FRCSC, FACOG<br />
Mehrnoosh Faghih, MD, FRCSC, FACOG<br />
Marc Anthony Fischer, BSc (Hons), MD, CFP, FRCSC<br />
D. Ray Freebury, MBChB, D(Obst)RCOG, FRCPC, DFAPA<br />
H. A. Pattinson, MBChB, FRCOG, FRCSC (Windsor)<br />
Our patient referral base includes Burlington, Hamilton, Guelph, Kitchener, Cambridge, Windsor,<br />
Niagara, Oakville, and Mississauaga.<br />
90 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
RESOURCES<br />
American College of Embryology (ACE) transforms a diverse group of embryology<br />
practitioners into a uniformly trained group of professionals. ACE is your organization<br />
and your voice. Please join our effort to advance the practice of clinical embryology and<br />
establish standards for embryology care in the United States.<br />
American College of Embryology is offering certification for Embryology<br />
practitioners at the following levels:<br />
Embryologist (EMB)<br />
Embryology Associate (EMA)<br />
Embryology Technologist (EMT)<br />
Certification requires theoretical and practical examinations.<br />
The College also offers grandfathering on a limited basis for qualified candidates.<br />
Please join the College and be<strong>com</strong>e certified Embryology practitioner!<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
91
CONFERENCES<br />
IVFonline Workshops on the Use of the<br />
global ® Blastocyst Vitrification and Thawing Kits – Based on S 3<br />
The success rates of recently developed vitrification methods for the<br />
cryopreservation of human oocytes and embryos have been highly<br />
encouraging, and vitrification is now widely applied in human IVF<br />
clinics. Vitrification of embryos increases the change of a pregnancy<br />
following cryopreservation, post-warming survival rates are shown to<br />
be as high as 90% which are significantly better than those achieved<br />
with slow freezing methods (Vajta et al., 2009, Stachecki et al., 2008).<br />
These long-waited results allow clinicians and embryologists to opt<br />
for other strategies such as single embryo transfer and vitrification of<br />
the remaining embryos, hence reducing multiple pregnancies and<br />
their associated consequences.<br />
LifeGlobal has recently developed kits for blastocyst vitrification and<br />
thawing based on the S 3 system developed by James Stachecki<br />
(Stachecki et al., 2008; Stachecki and Cohen, 2008) The S 3 system uses<br />
a <strong>com</strong>bination of ethylene glycol and glycerol as the cryoprotectants,<br />
avoiding the toxic effect of DMSO. The embryos are held in standard<br />
0.25-0.30 cc straws and thus not require any specific device. But<br />
undoubtedly, its most important features are that no special skills are<br />
required and that there is considerably more time to perform all steps<br />
of this method, making it a less stressful procedure that can be<br />
performed by all laboratory staff. Using this method, a pregnancy rate<br />
per transfer of 56 % has been reported (Stachecki et al. 2008).<br />
In order to demonstrate this technique, IVFonline has conducted<br />
several demonstrations and workshops in Europe in 2010. Nathalie<br />
Boonen, IVFonline Product Manager, and Dr. Ana Sousa Lopes,<br />
clinical and research adviser, coordinated the organization of these<br />
workshops, in conjunction with the meetings of the British Fertility<br />
Society, Bristol, UK., the Mediterranean Society for Reproductive<br />
Medicine, Budva, Montenegro, and The 1st International Congress on<br />
Controversies in Cryopreservation of Stem Cells, Reproductive Cells,<br />
Tissue and Organs, Valencia, Spain.<br />
Montenegro Workshop – May 6, 2010<br />
Danilo I Hospital Cetinje<br />
Montenegro Workshop – May 6, 2010<br />
Danilo I Hospital Cetinje<br />
The Montenegro workshop was held in the IVF unit of the Hospital of<br />
Cetinje and attracted 19 participants from the Balkan countries. In the<br />
morning session, Dr. Lopes presented an overview of the use of the<br />
global ® Blastocyst Vitrification and Thawing Kits – Based on S 3 ,<br />
followed by a practical demonstration to introduce all of the steps in<br />
detail. In the afternoon, the attendees were divided into four working<br />
groups for hands-on training. The interest of the attendees for this<br />
technique was clearly demonstrated by the questions raised<br />
throughout the day.<br />
The workshops in Spain were organized with the Fundación Jimenez<br />
Díaz, Madrid, the IVF unit of the Hospital De la Fé, Valencia, and the<br />
Clinic El Pilar, San Sebastian. Dr. Lopes gave the presentations and<br />
Madrid Workshop – May 26, 2010<br />
Fundación Jiménez Diaz<br />
92 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
CONFERENCES<br />
If you are interested to know when the next vitrification S 3 workshop<br />
will take place in or around your country, please<br />
contact Nathalie Boonen: nathalie@IVFonline.<strong>com</strong><br />
demonstrations in Madrid and Valencia. Dr. James Stachecki gave the<br />
presentation and demonstration in San Sebastian. A total of 39<br />
participants was present in the 3 workshops, representing 22 Spanish<br />
IVF clinics.<br />
The objective of these workshops is to introduce the participants to the<br />
principle behind S3 blastocyst vitrification system, and to give them<br />
the opportunity to see all steps of the procedure. With this background,<br />
they are able to proceed with the testing of the global ® Blastocyst<br />
Vitrification and Thawing Kits – Based on S 3 in their own IVF units.<br />
Valencia Workshop – May 27, 2010<br />
Hospital Universitario La Fe de Valencia<br />
The overall response of the attendees was very positive and it was<br />
generally agreed that the methods for use of the global ® Blastocyst<br />
Vitrification and Thawing Kits – Based on S 3 are simple and easy to<br />
perform. Vitrification offers a solution for embryo cryopreservation<br />
and is about to be<strong>com</strong>e a <strong>com</strong>mon part of the everyday routine in a<br />
human embryo laboratory, IVFonline encourages anyone interested in<br />
receiving training with the global ® Blastocyst Vitrification and<br />
Thawing Kits – Based on S 3 to contact us about joining one of our<br />
future workshops.<br />
References<br />
Valencia Workshop – May 27, 2010<br />
Hospital Universitario La Fe de Valencia<br />
Nathalie Boonen, Dr Ana S Lopes-Ana Ortiz,<br />
Dr Gloria Calderon, Dr Pedro Fernandez<br />
Stachecki, J.J., Cohen, J., (2008) S 3 Vitrifcation System: A novel approach to<br />
blastocyst freezing. J. Clin. Embryol. 11, 5-14.<br />
Stachecki, J.J., Garrisi, J., Sabino, S., Caetano, J.P., Wiemer, K.E., Cohen, J.,<br />
(2008) A new safe, simple and successful vitrification method for bovine<br />
and human blastocysts. Reprod Biomed Online 17, 360-367.<br />
Vajta, G., Nagy, Z.P., Cobo, A., Conceicao, J., Yovich, J., (2009) Vitrification in<br />
assisted reproduction: myths, mistakes, disbeliefs and confusion. Reprod<br />
Biomed Online 19 Suppl 3, 1-7.<br />
San Sebastian Workshop – June 1, 2010<br />
Centro Sanitario Virgen del Pilar<br />
San Sebastian Workshop – June 1, 2010<br />
Centro Sanitario Virgen del Pilar<br />
Dr James Stachecki<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
93
CONFERENCES<br />
A Successful Workshop presented by IVFonline/LifeGlobal<br />
West Coast Workshop held on January 23, 2010<br />
global Blastocyst Vitrification System – Based on S3<br />
On Saturday January 23, 2010 IVFonline held its first hands on S3<br />
Vitrification workshop at the Pacific Fertility Center in San Francisco. It<br />
was a one day workshop where participants were able to gain hands on<br />
experience working with the global® Blastocyst Vitrification System –<br />
Based on S3 and gain CEU’s in the process. We had a fantastic turn out at<br />
the event, with 21 West Coast participants. The response to the workshop<br />
invitation was so positive; it necessitated a waiting list be started to<br />
ac<strong>com</strong>modate potential attendees.<br />
Speaking at this workshop were Jacques Cohen, PhD, Klaus Wiemer, PhD<br />
and Don Rieger, PhD. Dr. Cohen gave an overview and spoke about the<br />
principals of the global® Blastocyst Vitrification System – Based on S3. Dr. Wiemer shared his own experience with<br />
the global® Blastocyst Vitrification System – Based on S3 and provided some of his data. Afterwards Dr. Wiemer<br />
demonstrated a “vitrification and devitrification” procedure. Participants then broke into small groups and went to one<br />
of the five stations to practice vitrifying and devitrifying mouse blastocysts with the global® Blastocyst Vitrification<br />
System – Based on S3. After the workshop IVFonline/LifeGlobal offered to send practice kits to all participating centers.<br />
The response and feedback from the workshop was tremendous.<br />
“I was very happy with the way the course went. I thought it was really excellent.”<br />
“Thank you for providing the educational activity for us. We look forward to the next one.”<br />
“Thanks for the workshop! You all did a stellar job and I learned a lot.”<br />
“I had a wonderful time and learned a lot! I especially loved the small, intimate setting and the one-on-one time.”<br />
A special thank you goes to Joe Conaghan PhD. and the Pacific Fertility Center who hosted the workshop at their<br />
beautiful education center. IVFonline provided breakfast goodies and lunch. Also, this workshop was an AAB-PEER<br />
approved event allowing participants to earn 0.5 CEU’s for attending.<br />
IVFonline has plans to hold more of these workshops this year. If you are interested in attending please contact us at<br />
sales@IVFonline.<strong>com</strong> and place your name on a list for one of our future workshops; no registration fee required.<br />
94 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
CONFERENCES<br />
Wel<strong>com</strong>e to PCRS<br />
2011 Annual Meeting, April 13 to 17<br />
Rancho Las Palmas<br />
41-000 Bob Hope Drive<br />
Rancho Mirage, CA 92270<br />
www.pcrsonline.org<br />
ASRM 2010<br />
American Society for Reproductive Medicine<br />
66th Annual Meeting<br />
"“Taking Reproduction to New Heights"<br />
October 23-27, 2010<br />
Colorado Convention Center<br />
Denver, Colorado<br />
www.asrm.org<br />
WWW.FERTMAG.COM • VOLUME <strong>12</strong> • FERTILITY MAGAZINE<br />
95
CONFERENCES<br />
AAB’s 2011 Annual Meeting and Educational<br />
Conference/CRB Symposium/NILA Meeting<br />
May <strong>12</strong>-14, 2011<br />
Hyatt Regency Austin<br />
Austin,Texas<br />
www.aab.org<br />
96 FERTILITY MAGAZINE • VOLUME <strong>12</strong> • WWW.FERTMAG.COM
Introducing the Coda ® 2<br />
Proven Filtration Technology to remove contaminants such as Volatile Organic<br />
Contaminants (VOCs) inside incubators<br />
…Common Sense Safety Devices to Protect Embryos, Stem Cells or any other Cell Culture<br />
The Coda ® Incubator Unit has proven itself in human IVF for the past two decades to remove Volatile Organic<br />
Contaminants (VOCs) presents in any incubator or laboratory.<br />
Coda ® 2 Kit<br />
Placed on the top shelf of the incubator<br />
Coda ® Unit<br />
Placed on the top shelf of<br />
the incubator<br />
Coda ® SP<br />
Mounted into the access<br />
hole of your incubator<br />
Coda ® 2 Kit<br />
C2KT-107<br />
C2KT-106 (w/o Coda® Xtra Inline® Filter)<br />
Available as an economical start up Kit for incubator<br />
and gas line: with 6 months supply of Coda ® Filters,<br />
1 Coda ® Xtra Inline ® Filter and accessories.<br />
CodaInside<br />
Coda ® Inside your incubator<br />
has proven to remove VOCs<br />
and CACs. Particulates that can<br />
potentially inhibit any cell<br />
culture development. Testing in<br />
certain laboratories has shown<br />
that in the presence of<br />
formaldahydes even the mouse cell culture was inhibited. Coda ® is a<br />
patented technology, manufactured by a certified ISO medical <strong>com</strong>pany and each building material is tested<br />
for any off-gassing and safe use in human embryos. Coda ® includes activated carbon filtration and a 99.997<br />
% HEPA filter to remove VOCs and CACs and particulates <strong>com</strong>pletely from the air inside your environment.<br />
This will improve your embryo and stem cell quality and development, along with giving you better overall<br />
performance and results. Our Coda ® 2 is ‘greener’, saves energy and is made of 100% recyclable materials.<br />
Do you know what VOCs reside inside your incubators<br />
styrene, methylcyclohexane, acetone, benzene, n-Decane, octane, etc.<br />
Coda …the only solution introduced since 1997<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
email: sales@IVFonline.<strong>com</strong><br />
FDA 510(k) Cleared
Introducing LGPS<br />
FDA 510(k) Cleared<br />
LifeGlobal ® Protein Supplement<br />
LifeGlobal ® Protein Supplement<br />
with the enhanced performance<br />
provided by α- & β-globulins.<br />
• Tested lot-to-lot<br />
• Stringent quality control and testing<br />
• One-cell Mouse Embryo Assay Testing<br />
• <strong>12</strong>-month shelf life<br />
• pH, Osmolality and Endotoxin Tested<br />
• High quality of manufacturing<br />
• ISO 13485:2003 & 9001:2000 Certified<br />
Product Description Features and Highlights Size Cat. #<br />
LifeGlobal ® Protein Supplement with the enhanced 20 ml LGPS-020<br />
LifeGlobal ® Protein Supplement (LGPS) performance provided by α- & β-globulins. Protein 50 ml LGPS-050<br />
supplement for in-vitro culture media.<br />
250 ml LGPS-250<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
email: sales@LifeGlobal.<strong>com</strong>
Coda ® Xtra Inline ® Filters<br />
Improved, more effective and economical due to an<br />
increased size and amount of pure filtering materials, with<br />
more amplified residence contact time and absorption<br />
capacity.<br />
Your first line of defense in removing contaminants such<br />
as Volatile Organic Contaminants (VOCs) and Chemical<br />
Air Contaminants (CACs) from in<strong>com</strong>ing gas sources.<br />
Coda ® Inline ® Filters have proven over 10 years to be safe and<br />
effective in Human IVF in purifying the gas for incubators,<br />
reducing contaminants and helping to improve results.<br />
The Coda ® Xtra Inline ® Filter protects the air and environment inside your incubator<br />
from harmful contaminants present in your gas sources ending up in your incubator,<br />
by removing all Volatile Organic Contaminants (VOCs), Chemical Air Contaminants<br />
(CACs) and particulates which may be in the gas or reside in the old tanks and<br />
tubing.<br />
Coda ® Inline ® Filters are the ‘only’ filter proven Safe and Effective in IVF and Human<br />
Reproduction.<br />
Coda ® Xtra Inline ® Filters are a proven quality product.<br />
• Your first line of defense in removing contaminants such as Volatile Organic<br />
Contaminants (VOCs) and Chemical Air Contaminants (CACs) from in<strong>com</strong>ing<br />
gas sources.<br />
• Contains 100% clean activated carbon and a 99.997% HEPA filter system.<br />
• Made in the USA with tested proven materials.<br />
• Proven in over 600 labs worldwide for 15+ years in stem cell research, hES<br />
cells, embryonic development and human reproduction and IVF.<br />
• Human IVF Laboratories that use our Coda ® Inline ® Filters have reported<br />
better and more consistant overall pregnancy rates and increases in live birth<br />
rates.<br />
Coda ® Xtra Inline ® Filters – Small Investments – Big Savings<br />
• Attaches to your in<strong>com</strong>ing gas lines supplying cleaner gas due to an<br />
increased amount of pure filtering materials, with an amplified residual contact<br />
time and absorption capacity.<br />
• Manufactured in a clean room environment with stringent quality control.<br />
• Delivered to you in an air tight sealed, sterilized packages.<br />
• Economical and easy to install.<br />
• Our Patented Technology makes Coda ® Filters a superior product and is<br />
Patented Protected in the United States, European Union, Australia and other<br />
countries worldwide. Coda ® Inline ® Filters are covered by the following<br />
patents: U.S. Patent No. 6,013,119, No. 6,843,818, No. 6,225,110 and No.<br />
6,200,362. The European Community Patent No: 98 923 448.9.<br />
N.S.<br />
Control<br />
Coda<br />
N.S.<br />
P < 0.05<br />
P < 0.05<br />
FDA 510(k) Cleared<br />
Be aware of counterfeit Inline ® Filters not safe for use in Human IVF.<br />
You may introduce more contaminants as filters may not be manufactured<br />
in a clean room environment and filtering materials may not be cleaned,<br />
tested and properly qualified for safe use in Human IVF.<br />
Grade 1<br />
Day 7<br />
Blasts<br />
Fresh Frozen<br />
Day 8 Pregnant (Day 90+)<br />
Effect of Coda Filtration on Bovine Embryo Qualuty and Pregancy Rates<br />
(Merton et al., Theriogenology 67, <strong>12</strong>33-<strong>12</strong>38, 2007)
A Unified Approach to Human Embryo Culture<br />
More than 20 independent studies with published results on<br />
global ® medium.<br />
• Based on global ® success<br />
• Minimizes stress to the embryo<br />
• Same chemical environment<br />
throughtout all stages of oocyte<br />
and embryo handling and culture<br />
• Better embryo development<br />
• Easy to use<br />
• High Quality of Manufacturing<br />
• Stringent Quality Control<br />
• Fresh Delivery<br />
• ISO 13485:2003 & 9001:2000 Certified<br />
US/Canada: 1-800-720-6375<br />
International: 1-519-826-5800<br />
Fax: 1-519-826-6947<br />
email: sales@LifeGlobal.<strong>com</strong>