Teacher's notes and answers to questions in the book - Hodder Plus ...
Teacher's notes and answers to questions in the book - Hodder Plus ...
Teacher's notes and answers to questions in the book - Hodder Plus ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
WJEC GCSE Additional Science Teacher’s Notes<br />
14. How is water formed from hydrogen <strong>and</strong> oxygen<br />
The oxygen a<strong>to</strong>m shares one electron from one hydrogen a<strong>to</strong>m <strong>and</strong> ano<strong>the</strong>r electron from a<br />
second hydrogen a<strong>to</strong>m.<br />
15. Why is water a liquid at room temperature<br />
The freez<strong>in</strong>g po<strong>in</strong>t of pure water is 0 °C <strong>and</strong> its boil<strong>in</strong>g po<strong>in</strong>t is 100 °C, room temperature<br />
(10–20 °C) is between <strong>the</strong>se two values hence water is a liquid at <strong>the</strong>se temperatures.<br />
16. Why is water only a poor conduc<strong>to</strong>r of electricity<br />
There are few H + <strong>and</strong> OH – ions <strong>in</strong> pure water – hence <strong>the</strong>re are few charged particles free <strong>to</strong><br />
move <strong>and</strong> so water is a poor conduc<strong>to</strong>r of electricity.<br />
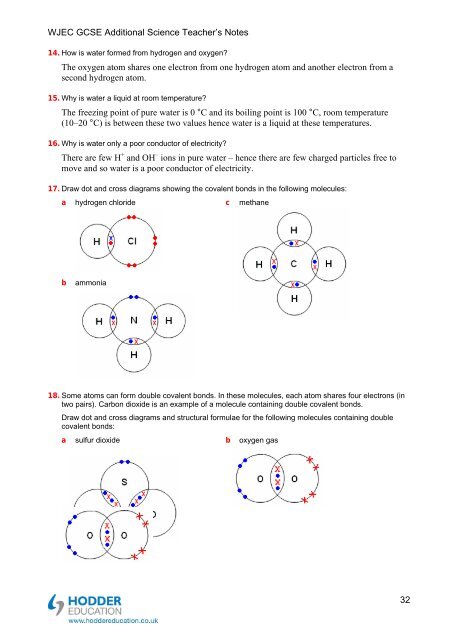
17. Draw dot <strong>and</strong> cross diagrams show<strong>in</strong>g <strong>the</strong> covalent bonds <strong>in</strong> <strong>the</strong> follow<strong>in</strong>g molecules:<br />
a hydrogen chloride c methane<br />
b ammonia<br />
18. Some a<strong>to</strong>ms can form double covalent bonds. In <strong>the</strong>se molecules, each a<strong>to</strong>m shares four electrons (<strong>in</strong><br />
two pairs). Carbon dioxide is an example of a molecule conta<strong>in</strong><strong>in</strong>g double covalent bonds.<br />
Draw dot <strong>and</strong> cross diagrams <strong>and</strong> structural formulae for <strong>the</strong> follow<strong>in</strong>g molecules conta<strong>in</strong><strong>in</strong>g double<br />
covalent bonds:<br />
a sulfur dioxide<br />
b oxygen gas<br />
32