Single Vial Docetaxel Injection - ION Solutions
Single Vial Docetaxel Injection - ION Solutions
Single Vial Docetaxel Injection - ION Solutions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
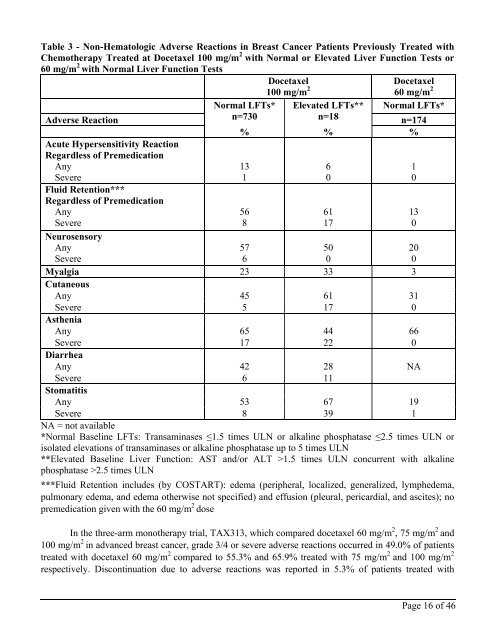
Table 3 - Non-Hematologic Adverse Reactions in Breast Cancer Patients Previously Treated with<br />
Chemotherapy Treated at <strong>Docetaxel</strong> 100 mg/m 2 with Normal or Elevated Liver Function Tests or<br />
60 mg/m 2 with Normal Liver Function Tests<br />
<strong>Docetaxel</strong><br />
<strong>Docetaxel</strong><br />
100 mg/m 2 60 mg/m 2<br />
Adverse Reaction<br />
Acute Hypersensitivity Reaction<br />
Regardless of Premedication<br />
Any<br />
Severe<br />
Fluid Retention***<br />
Regardless of Premedication<br />
Any<br />
Severe<br />
Neurosensory<br />
Any<br />
Severe<br />
Normal LFTs* Elevated LFTs** Normal LFTs*<br />
n=730<br />
n=18 n=174<br />
% % %<br />
13<br />
1<br />
56<br />
8<br />
57<br />
6<br />
50<br />
0<br />
20<br />
0<br />
Myalgia 23 33 3<br />
Cutaneous<br />
Any 45 61 31<br />
Severe 5 17 0<br />
Asthenia<br />
Any 65 44 66<br />
Severe 17 22 0<br />
Diarrhea<br />
Any 42 28 NA<br />
Severe 6 11<br />
Stomatitis<br />
Any 53 67 19<br />
Severe 8 39 1<br />
NA = not available<br />
*Normal Baseline LFTs: Transaminases ≤1.5 times ULN or alkaline phosphatase ≤2.5 times ULN or<br />
isolated elevations of transaminases or alkaline phosphatase up to 5 times ULN<br />
**Elevated Baseline Liver Function: AST and/or ALT >1.5 times ULN concurrent with alkaline<br />
phosphatase >2.5 times ULN<br />
***Fluid Retention includes (by COSTART): edema (peripheral, localized, generalized, lymphedema,<br />
pulmonary edema, and edema otherwise not specified) and effusion (pleural, pericardial, and ascites); no<br />
premedication given with the 60 mg/m 2 dose<br />
In the three-arm monotherapy trial, TAX313, which compared docetaxel 60 mg/m 2 , 75 mg/m 2 and<br />
100 mg/m 2 in advanced breast cancer, grade 3/4 or severe adverse reactions occurred in 49.0% of patients<br />
treated with docetaxel 60 mg/m 2 compared to 55.3% and 65.9% treated with 75 mg/m 2 and 100 mg/m 2<br />
respectively. Discontinuation due to adverse reactions was reported in 5.3% of patients treated with<br />
6<br />
0<br />
61<br />
17<br />
1<br />
0<br />
13<br />
0<br />
Page 16 of 46