A Guide to HPLC & LC-MS Buffer Selection - by John W ... - Hplc.eu
A Guide to HPLC & LC-MS Buffer Selection - by John W ... - Hplc.eu
A Guide to HPLC & LC-MS Buffer Selection - by John W ... - Hplc.eu
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Using <strong>Buffer</strong>s with <strong>HP<strong>LC</strong></strong> and <strong>LC</strong>-<strong>MS</strong><br />
When samples contain ionizable compounds, the mobile phase pH can be one of<br />
the most important variables in the control of retention in a reversed-phase<br />
<strong>HP<strong>LC</strong></strong> (RP-<strong>HP<strong>LC</strong></strong>) separation. However, if it is not controlled properly, pH can<br />
be a source of many problems. Since most compounds analyzed <strong>by</strong> RP-<strong>HP<strong>LC</strong></strong><br />
contain one or more acidic or basic functional groups, most mobile phases<br />
require pH control. For this reason, buffers are widely used. This booklet<br />
highlights some of the important aspects of mobile phase pH for the practical<br />
chroma<strong>to</strong>grapher.<br />
Why Control pH<br />
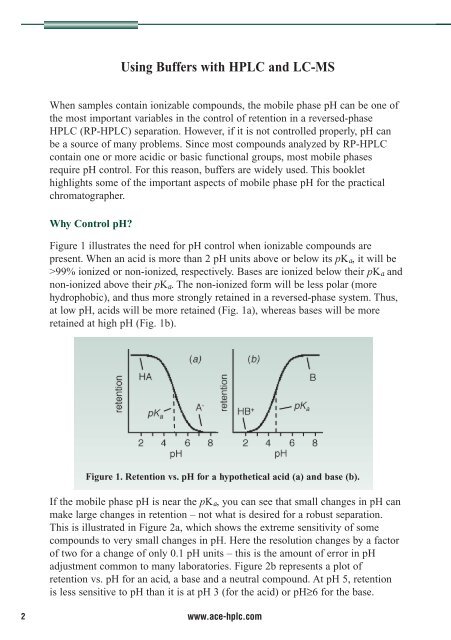
Figure 1 illustrates the need for pH control when ionizable compounds are<br />
present. When an acid is more than 2 pH units above or below its pK a , it will be<br />
>99% ionized or non-ionized, respectively. Bases are ionized below their pKa and<br />
non-ionized above their pKa. The non-ionized form will be less polar (more<br />
hydrophobic), and thus more strongly retained in a reversed-phase system. Thus,<br />
at low pH, acids will be more retained (Fig. 1a), whereas bases will be more<br />
retained at high pH (Fig. 1b).<br />
Figure 1. Retention vs. pH for a hypothetical acid (a) and base (b).<br />
If the mobile phase pH is near the pKa, you can see that small changes in pH can<br />
make large changes in retention – not what is desired for a robust separation.<br />
This is illustrated in Figure 2a, which shows the extreme sensitivity of some<br />
compounds <strong>to</strong> very small changes in pH. Here the resolution changes <strong>by</strong> a fac<strong>to</strong>r<br />
of two for a change of only 0.1 pH units – this is the amount of error in pH<br />
adjustment common <strong>to</strong> many labora<strong>to</strong>ries. Figure 2b represents a plot of<br />
retention vs. pH for an acid, a base and a n<strong>eu</strong>tral compound. At pH 5, retention<br />
is less sensitive <strong>to</strong> pH than it is at pH 3 (for the acid) or pH≥6 for the base.<br />
2 www.ace-hplc.com