Asteroid Spectroscopy
Asteroid Spectroscopy
Asteroid Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
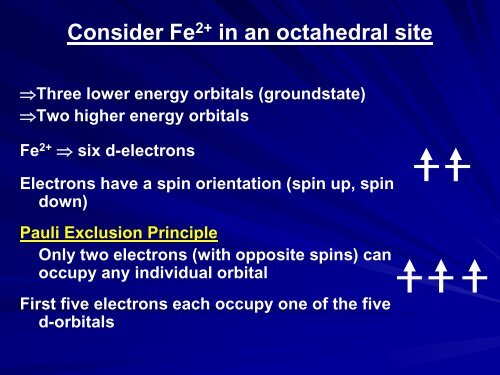
Consider Fe 2+ in an octahedral site<br />
⇒Three lower energy orbitals (groundstate)<br />
⇒Two higher energy orbitals<br />
Fe 2+ ⇒ six d-electrons<br />
Electrons have a spin orientation (spin up, spin<br />
down)<br />
Pauli Exclusion Principle<br />
Only two electrons (with opposite spins) can<br />
occupy any individual orbital<br />
First five electrons each occupy one of the five<br />
d-orbitals