Fluid balance and electrolyte distribution in human body.
Fluid balance and electrolyte distribution in human body.
Fluid balance and electrolyte distribution in human body.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Fluid</strong> <strong>balance</strong> <strong>and</strong> <strong>electrolyte</strong> <strong>distribution</strong><br />
<strong>in</strong> <strong>human</strong> <strong>body</strong>.<br />
Mass Percent<br />
• other way of describ<strong>in</strong>g the amount of solute <strong>in</strong> a solution<br />
• Describes what percentage of a solution by mass is<br />
comprised by solute.<br />
mass of solute<br />
mass of solute<br />
× 100 % =<br />
× 100%<br />
total mass of solution mass of solute + mass of solvent<br />
• Example<br />
A student prepares a solution from 5.00 g of sodium fluoride dissolved<br />
<strong>in</strong> 95.00 g of water.<br />
What is the mass percent of sodium fluoride
Us<strong>in</strong>g Moles to Describe the Amount of<br />
Substance <strong>in</strong> a Solution<br />
• A number of units may be used to describe the<br />
concentration of a solute <strong>in</strong> a solution.<br />
• The most common unit is molarity (M).<br />
• The Molarity of a solution is equal to the moles of solute<br />
divided by the total volume of the solution.<br />
M<br />
=<br />
moles solute<br />
L solution<br />
=<br />
molar<br />
mass<br />
mass<br />
( g)<br />
solute<br />
solute x L solution<br />
• If we know the mass of solute we dissolved <strong>in</strong> the<br />
solution, we can convert the mass the solute to moles of<br />
solute <strong>and</strong> calculate the molarity.<br />
• The Molality of a solution is equal to the moles of solute<br />
per kilogram of solvent<br />
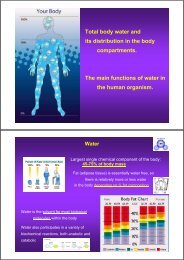
Total <strong>body</strong> water <strong>and</strong><br />
its <strong>distribution</strong> <strong>in</strong> the <strong>body</strong><br />
compartments.<br />
The ma<strong>in</strong> functions of water <strong>in</strong><br />
the <strong>human</strong> organism.
Water<br />
Largest s<strong>in</strong>gle chemical component of the <strong>body</strong>:<br />
45-75% of <strong>body</strong> mass<br />
Fat (adipose tissue) is essentially water free, so<br />
there is relatively more or less water<br />
<strong>in</strong> the <strong>body</strong> depend<strong>in</strong>g on % fat composition<br />
Water is the solvent for most biological<br />
molecules with<strong>in</strong> the <strong>body</strong><br />
Water also participates <strong>in</strong> a variety of<br />
biochemical reactions, both anabolic <strong>and</strong><br />
catabolic<br />
Body fat measur<strong>in</strong>g<br />
Sk<strong>in</strong>fold Caliper<br />
http://www.l<strong>in</strong>ear-software.com/onl<strong>in</strong>e.html
<strong>Fluid</strong> Compartments<br />
Body <strong>Fluid</strong>s are separated by semi-permeable membranes <strong>in</strong>to various<br />
physiological (functional) compartments<br />
• Two Compartment Model<br />
- Intracellular = Cytoplasmic (<strong>in</strong>side cells)<br />
- Extracellular (outside cells)<br />
The Two Compartment Model is useful cl<strong>in</strong>ically for underst<strong>and</strong><strong>in</strong>g the<br />
<strong>distribution</strong> of many drugs <strong>in</strong> the <strong>body</strong><br />
• Three Compartment Model<br />
– [1] Intracellular = Cytoplasmic (<strong>in</strong>side cells)<br />
– Extracellular compartment is subdivided <strong>in</strong>to:<br />
• [2] Interstitial = Intercellular = Lymph (between the cells <strong>in</strong> the tissues)<br />
• [3] Plasma (fluid portion of the blood)<br />
The Three Compartment Model is more useful for underst<strong>and</strong><strong>in</strong>g<br />
physiological processes<br />
Other models with more compartments can sometimes be useful, e.g., consider lymph <strong>in</strong> the<br />
lymph vessels, CSF, ocular fluids, synovial <strong>and</strong> serous fluids as separate compartments<br />
<strong>Fluid</strong> Compartments<br />
• Total Body Water (TBW) - 42L,<br />
60% of <strong>body</strong> weight<br />
– Intracellular <strong>Fluid</strong> (ICF) -<br />
28L, 67% of TBW<br />
– Extracellular <strong>Fluid</strong> (ECF) -<br />
14L, 33% of TBW<br />
• Interstitial <strong>Fluid</strong> - 11L,<br />
80% ECF<br />
• Plasma - 3L, 20% of<br />
ECF
Water <strong>balance</strong><br />
– Sources for 2500 ml<br />
- average daily<br />
<strong>in</strong>take<br />
• Metabolic Water<br />
• Preformed Water<br />
– Ingested Foods<br />
– Ingested Liquids<br />
– Balance achieved if<br />
daily output also =<br />
2500 ml<br />
• GI tract<br />
• Lungs<br />
• Sk<strong>in</strong><br />
– evaporation<br />
– perspiration<br />
• Kidneys<br />
Water Movement Between the ICF <strong>and</strong> ECF<br />
Osmolality – the concentrations of solutes <strong>in</strong> water<br />
– solutes will <strong>in</strong>fluence the movement of water across membranes<br />
H 2<br />
O<br />
π = iRTc<br />
Aquapor<strong>in</strong>s- water channel prote<strong>in</strong>s <strong>in</strong> membranes<br />
Net filtration (Starl<strong>in</strong>g hypothesis)<br />
= forces favor<strong>in</strong>g filtration – forces oppos<strong>in</strong>g filtration<br />
As fluid flows through capillary it looses water <strong>and</strong> create greater osmotic<br />
return of water as it flows toward ve<strong>in</strong>ule end of capillary<br />
Forces favor<strong>in</strong>g filtration<br />
- Capillary hydrostatic pressure (blood pressure)<br />
- Interstitial oncotic pressure (water-pull<strong>in</strong>g)<br />
Forces favor<strong>in</strong>g reabsorption<br />
- Plasma oncotic pressure (water-pull<strong>in</strong>g)<br />
- Interstitial hydrostatic pressure<br />
H 2<br />
O<br />
filtration<br />
H 2<br />
O<br />
reabsorption
Oncotic pressure…Colloid osmotic pressure<br />
• is formed by colloid particles dissolved <strong>in</strong> solution<br />
• <strong>in</strong> plasma the major part forms prote<strong>in</strong>s 65-85 g/l<br />
Electrophoretic separation of plasma prote<strong>in</strong>s<br />
(directly proportional to size <strong>and</strong> charge)<br />
Album<strong>in</strong><br />
60%<br />
α 1<br />
globul<strong>in</strong> 1-antitryps<strong>in</strong>, 1-acid glycoprote<strong>in</strong><br />
α 2<br />
globul<strong>in</strong> haptoglob<strong>in</strong>, 2-macroglobul<strong>in</strong>,<br />
2-antiplasm<strong>in</strong>, ceruloplasm<strong>in</strong><br />
4% 7%<br />
β globul<strong>in</strong><br />
transfer<strong>in</strong>, complement, LDL<br />
10%<br />
fibr<strong>in</strong>ogen<br />
γ globul<strong>in</strong> = Imunoglobul<strong>in</strong>s<br />
IgA, IgD, IgE, IgG <strong>and</strong> IgM<br />
Mr 67x10 3 Mr150x10 3<br />
Mr340x10 3<br />
5% 14%<br />
Water Movement Between the ICF <strong>and</strong> ECF<br />
20L/day<br />
18L/day
Osmotic Equilibrium<br />
Plasma Osmolarity - Measures ECF Osmolarity<br />
• Plasma is cl<strong>in</strong>ically accessible<br />
• Dom<strong>in</strong>ated by [Na + ] <strong>and</strong> the associated anions<br />
• Under normal conditions, ECF osmolarity can be roughly estimated<br />
as:<br />
P OSM = 2 [Na + ] p<br />
……..270-300 mOsm<br />
{ P OSM = 2[Na + ] + 2[K + ] + [Urea] + [Glucose] }
Edema<br />
Accumulation of fluid with<strong>in</strong> the<br />
<strong>in</strong>terstitial spaces<br />
• Causes:<br />
– Increase <strong>in</strong> hydrostatic pressure (blood pressure / hypertension)<br />
– Losses or dim<strong>in</strong>ished production of plasma album<strong>in</strong> (hypoprote<strong>in</strong>emia<br />
…decrease <strong>in</strong> oncotic pressure /malnutrition (at <strong>in</strong>sufficient supply of prote<strong>in</strong>s<br />
…abdom<strong>in</strong>al edema/ <strong>in</strong>sufficient production of prote<strong>in</strong>s at cirrhosis/ large<br />
losses of prote<strong>in</strong>s by kidney at nephrotic syndrome/)<br />
- Increases <strong>in</strong> capillary permeability (at anaphylaxis, allergic<br />
reaction (release of histam<strong>in</strong>), <strong>in</strong>flammation)<br />
- Lymph obstruction – elephantitus, flibitus<br />
- Decreased resorption due to raised systemic venous<br />
pressure – edema due to heart failure<br />
Edema
Regulat<strong>in</strong>g <strong>Fluid</strong> Intake<br />
Thirst<br />
Thirst Quench<strong>in</strong>g<br />
1. 2.<br />
Wett<strong>in</strong>g the oral mucosa<br />
(temporary)<br />
Stretch<strong>in</strong>g of the stomach<br />
Decreased blood/<strong>body</strong><br />
fluid osmolarity =<br />
<strong>in</strong>creased hydration<br />
(dilution) of the blood is<br />
the most important<br />
Regulation of <strong>Fluid</strong> Output<br />
• Hormonal control<br />
– 1 Antidiuretic hormone (ADH) [neurohypophysis]<br />
– 2 Aldosterone [adrenal cortex]<br />
– 3 Atrial natriuretic peptide (ANP) [heart atrial walls]<br />
• Causes of physiologic fluid im<strong>balance</strong>s<br />
– Dehydration: ↓ blood pressure, ↓ GFR<br />
– Overhydration: ↑ blood pressure, ↑ GFR<br />
– Hyperventilation - water loss through lungs<br />
– Vomit<strong>in</strong>g & Diarrhea - excessive water loss<br />
– Fever - heavy perspiration<br />
– exudat<strong>in</strong>g Burns, contusion - fluid loss<br />
– Hemorrhage – if blood loss is severe
Atrial natriuretic peptide (ANP) is a 28-am<strong>in</strong>o acid peptide that is synthesized, stored, <strong>and</strong><br />
released by atrial myocytes <strong>in</strong> response to atrial distension<br />
- elevated levels of ANP are found dur<strong>in</strong>g hypervolemic states (elevated blood volume) <strong>and</strong><br />
congestive heart failure<br />
A second natriuretic peptide (bra<strong>in</strong>-type natriuretic peptide; BNP) is a 32-am<strong>in</strong>o acid peptide that<br />
is synthesized with<strong>in</strong> the ventricles (as well as <strong>in</strong> the bra<strong>in</strong> where it was first identified). Like<br />
ANP, BNP is released by the same mechanisms that release ANP, <strong>and</strong> it has similar<br />
physiological actions, BNP serves as sensitive, diagnostic markers for heart failure <strong>in</strong><br />
patients<br />
Regulation of <strong>Fluid</strong> Output
Osm V PB<br />
ADH<br />
Factors affect<strong>in</strong>g<br />
ADH release<br />
Ur<strong>in</strong>e osmolarity regulation by ADH<br />
ADH
Human angiotens<strong>in</strong>ogen<br />
is 118 am<strong>in</strong>o acids long<br />
Pathway of RAAS<br />
Pr<strong>in</strong>cipal cells & aldosterone
Atrial natriuretic peptide<br />
28-am<strong>in</strong>o acid peptide<br />
Distribution of Solutes<br />
Interstitial fluid is<br />
essentially an ultrafiltrate<br />
of plasma,<br />
water <strong>and</strong> <strong>electrolyte</strong>s move freely with<strong>in</strong><br />
this compartment <strong>and</strong> between it <strong>and</strong> the<br />
<strong>in</strong>travascular fluid.<br />
Intravascular fluid has almost the same<br />
composition as <strong>in</strong>terstitial fluid except<br />
for its higher prote<strong>in</strong> level.
Electrolyte Balance<br />
Electrolytes have 4 important physiological functions <strong>in</strong> the <strong>body</strong><br />
• essential m<strong>in</strong>erals <strong>in</strong> certa<strong>in</strong> biochemical reactions<br />
• control osmosis = control the movement of water between<br />
compartments<br />
• ma<strong>in</strong>ta<strong>in</strong> acid-base <strong>balance</strong><br />
• conduct electrical currents (depolarization events)<br />
Regulators:<br />
Aldosterone ↑ [Na + ] [Cl - ] [H 2 O] ↓ [K + ]<br />
Atrial Natriuretic Peptide (opposite effect)<br />
Antidiuretic Hormone ↑ [H 2 O] (↓ [solutes])<br />
Parathyroid Hormone ↑ [Ca ++ ] ↓ [HPO 4- ]<br />
Calciton<strong>in</strong> (opposite effect)<br />
Female sex hormones ↑ [H 2 O]<br />
Electrolytes<br />
• Sodium (Na + ) - 136-146 mmol/liter<br />
– Most abundant cation<br />
• major ECF cation (90% of cations present)<br />
• determ<strong>in</strong>es osmolarity of ECF<br />
– Regulation<br />
• Aldosterone<br />
• ADH<br />
• ANP<br />
– Homeostatic im<strong>balance</strong>s<br />
• Hyponatremia<br />
• Hypernatremia
Hypertonic Alterations - Related to sodium ga<strong>in</strong> or water loss<br />
• Hypernatremia<br />
– Serum sodium >146 mmol/L<br />
– Water movement from the ICF to the ECF<br />
• Intracellular dehydration<br />
– Manifestations:<br />
• Convulsions, pulmonary edema, tachycardia, etc.<br />
• Water deficit<br />
- Dehydration<br />
- Renal free water clearance<br />
- Manifestations:<br />
– Tachycardia, weak pulses<br />
– Elevated hematocrit <strong>and</strong> serum sodium level<br />
Hypotonic Alterations - Related to Hyponatremia or free water excess<br />
• Hyponatremia<br />
- Serum sodium level
Electrolytes<br />
• Chloride (Cl - ) - 95-103 mmol/L<br />
– Major ECF anion<br />
• helps <strong>balance</strong> osmotic potential <strong>and</strong> electrostatic equilibrium<br />
between fluid compartments<br />
• plasma membranes tend to be leaky to Cl - anions<br />
– Regulation: aldosterone<br />
– Homeostatic im<strong>balance</strong>s<br />
• Hypochloremia - results <strong>in</strong> muscle spasms, coma (usually<br />
occurs with hyponatremia) often due to prolonged vomit<strong>in</strong>g<br />
(elevated sweat chloride diagnostic of Cystic Fibrosis)<br />
Electrolytes<br />
• Potassium (K + )<br />
– Major ICF cation, concentration ma<strong>in</strong>ta<strong>in</strong>ed by the Na + /K + pump<br />
• <strong>in</strong>tracellular 120-125 mmol/L<br />
• plasma 3.5-5.0 mmol/L<br />
– Very important role <strong>in</strong> rest<strong>in</strong>g membrane potential (RMP) <strong>and</strong> <strong>in</strong><br />
action potentials = essential for transmission <strong>and</strong> conduction of nerve<br />
impulses, normal cardiac rhythms, <strong>and</strong> skeletal <strong>and</strong> smooth muscle<br />
contraction<br />
• Changes <strong>in</strong> pH affect K + <strong>balance</strong><br />
– Hydrogen ions accumulate <strong>in</strong> the ICF dur<strong>in</strong>g states of acidosis. K +<br />
shifts out to ma<strong>in</strong>ta<strong>in</strong> a <strong>balance</strong> of cations across the membrane.<br />
• Aldosterone, <strong>in</strong>sul<strong>in</strong>, <strong>and</strong> catecholam<strong>in</strong>es <strong>in</strong>fluence serum potassium<br />
levels<br />
• Homeostatic im<strong>balance</strong>s<br />
• Hypokalemia<br />
• Hyperkalemia
• Hypokalemia<br />
- Potassium level 5.5 mmol/L<br />
- Caused by <strong>in</strong>creased <strong>in</strong>take, shift of K+ from ICF, decreased renal excretion,<br />
<strong>in</strong>sul<strong>in</strong> deficiency, or cell trauma<br />
- Mild attacks<br />
- Hypopolarized membrane, caus<strong>in</strong>g neuromuscular irritability, T<strong>in</strong>gl<strong>in</strong>g of lips<br />
<strong>and</strong> f<strong>in</strong>gers, restlessness, <strong>in</strong>test<strong>in</strong>al cramp<strong>in</strong>g, <strong>and</strong> diarrhea<br />
- Severe attacks<br />
- The cell is not able to repolarize, result<strong>in</strong>g <strong>in</strong> muscle weakness, loss or<br />
muscle tone<br />
Electrolytes<br />
• Calcium (Ca 2+ )<br />
– Most abundant ion <strong>in</strong> <strong>body</strong><br />
• plasma 2.3-2.6 mmol/L<br />
• most stored <strong>in</strong> bone (98%) as hydroxyapatite<br />
- Necessary for structure of bones <strong>and</strong> teeth, blood clott<strong>in</strong>g, hormone<br />
secretion, <strong>and</strong> cell receptor function<br />
- Regulation:<br />
• Parathyroid Hormone (PTH) - ↑ blood Ca 2+<br />
• Calciton<strong>in</strong> (CT) - ↓ blood Ca 2+<br />
– Homeostatic im<strong>balance</strong>s:<br />
• Hypocalcemia - muscle cramps, convulsions<br />
• Hypercalcemia - vomit<strong>in</strong>g, cardiovascular symptoms, coma;<br />
prolonged abnormal calcium deposition, e.g., stone<br />
formation
Electrolytes<br />
• Phosphate (H 2 PO 4- , HPO 4<br />
2-<br />
, PO 4<br />
3-<br />
)<br />
– Important ICF anions; plasma 1.7-2.6 mmol/L<br />
• most (85%) is stored <strong>in</strong> bone as calcium salts<br />
• also comb<strong>in</strong>ed with lipids, prote<strong>in</strong>s, carbohydrates, nucleic acids (DNA<br />
<strong>and</strong> RNA), <strong>and</strong> high energy phosphate transport compound<br />
• important acid-base buffer <strong>in</strong> <strong>body</strong> fluids<br />
– Regulation - regulated <strong>in</strong> an <strong>in</strong>verse relationship with Ca 2+ by PTH <strong>and</strong><br />
calciton<strong>in</strong> <strong>and</strong> Vitam<strong>in</strong> D (If the concentration of one <strong>in</strong>creases, that of the other<br />
decreases)<br />
– Parathyroid hormone (PTH) - Increases plasma calcium levels<br />
– Vitam<strong>in</strong> D = Fat-soluble steroid - Increases calcium absorption from the GI tract<br />
– Calciton<strong>in</strong> - Decreases plasma calcium levels<br />
– Homeostatic im<strong>balance</strong>s<br />
• Phosphate concentrations shift oppositely from calcium concentrations<br />
<strong>and</strong> symptoms are usually due to the related calcium excess or deficit<br />
Hypophosphatemia <strong>and</strong> Hyperphosphatemia<br />
• Hypophosphatemia<br />
– Osteomalacia (soft bones)<br />
– Muscle weakness<br />
– Bleed<strong>in</strong>g disorders (platelet impairment)<br />
– Anemia<br />
– Leukocyte alterations<br />
• Hyperphosphatemia<br />
– High phosphate levels are related to the low calcium levels<br />
- Increased neuromuscular excitability (partial depolarization)<br />
- Muscle cramps
Electrolytes<br />
• Magnesium (Mg 2+ )<br />
– 2 nd most abundant <strong>in</strong>tracellular <strong>electrolyte</strong>, 0.8-1.3 mmol/L <strong>in</strong> plasma<br />
• more than half is stored <strong>in</strong> bone, most of the rest <strong>in</strong> ICF<br />
(cytoplasm)<br />
• important enzyme cofactor; <strong>in</strong>volved <strong>in</strong> neuromuscular activity,<br />
nerve transmission <strong>in</strong> CNS, <strong>and</strong> myocardial function<strong>in</strong>g<br />
– Homeostatic im<strong>balance</strong><br />
• Hypomagnesemia - Associated with hypocalcemia <strong>and</strong><br />
hypokalemia, Neuromuscular irritability,Tetany, Convulsions,<br />
Hyperactive reflexes vomit<strong>in</strong>g, cardiac arrhythmias<br />
• Hypermagnesemia - Muscle weakness, Hypotension,<br />
Respiratory depression, Lethargy, drows<strong>in</strong>ess, Bradycardia<br />
Acid-Base Balance<br />
• Normal metabolism produces H + (acidity)<br />
• Three Homeostatic mechanisms:<br />
– Buffer systems - <strong>in</strong>stantaneous; temporary<br />
– Exhalation of CO 2 - operates with<strong>in</strong> m<strong>in</strong>utes; cannot<br />
completely correct serious im<strong>balance</strong>s<br />
– Kidney excretion - can completely correct any im<strong>balance</strong><br />
(eventually)<br />
• Buffer Systems<br />
– Consists of a weak acid <strong>and</strong> the salt of that acid which<br />
functions as a weak base
Acid-Base Balance<br />
• Carbonic Acid - Bicarbonate Buffer<br />
– A weak base (carbonic anhydrase)<br />
H + + HCO 3- ⇔ H 2 CO 3 ⇔ H 2 O + CO 2<br />
• Phosphate Buffer<br />
NaOH + NaH 2 PO 4 ⇔ H 2 O + Na 2 HPO 4<br />
HCl + Na 2 HPO 4 ⇔ NaCl + NaH 2 PO 4<br />
• Prote<strong>in</strong> Buffer (resp. hemoglob<strong>in</strong> & album<strong>in</strong>)<br />
Most abundant buffer <strong>in</strong> <strong>body</strong> cells <strong>and</strong> plasma<br />
Am<strong>in</strong>o acids have am<strong>in</strong>e group (proton<br />
acceptor = weak base) <strong>and</strong> a carboxyl group<br />
(proton donor = weak acid)<br />
Acid-Base Balance<br />
• CNS <strong>and</strong> peripheral<br />
chemoreceptors control<br />
changes <strong>in</strong> blood pH<br />
• Increased [H + ] causes<br />
immediate hyperventilation<br />
<strong>and</strong> later <strong>in</strong>creased renal<br />
secretion of [H + ] <strong>and</strong> [NH 4+ ]<br />
• Decreased [H + ] causes<br />
immediate hypoventilation<br />
<strong>and</strong> later decreased renal<br />
secretion of [H + ] <strong>and</strong> [NH 4+ ]
• Acidosis<br />
– High blood [H + ]<br />
– Low blood pH, 7.45<br />
Acid-Base Im<strong>balance</strong>s<br />
• Acid-Base im<strong>balance</strong>s may be due to problems with ventilation or due to a<br />
variety of metabolic problems<br />
– Respiratory Acidosis (pCO 2<br />
> 45 mm Hg)<br />
– Respiratory Alkalosis (pCO 2<br />
< 35 mm Hg)<br />
– Metabolic Acidosis (HCO 3-<br />
< 23 mmol/l)<br />
– Metabolic Alkalosis (HCO 3-<br />
> 26 mmol/l)<br />
• Compensation: the physiological response to an acid-base im<strong>balance</strong><br />
beg<strong>in</strong>s with adjustments by the system less <strong>in</strong>volved<br />
Causes of Acid-Base Im<strong>balance</strong>s<br />
• Respiratory Acidosis<br />
– Chronic Obstructive Pulmonary Diseases e.g., emphysema,<br />
pulmonary fibrosis<br />
– Pneumonia<br />
• Respiratory Alkalosis<br />
– Hysteria<br />
– Fever<br />
– Asthma
Causes of Acid-Base Im<strong>balance</strong>s<br />
• Metabolic Acidosis<br />
– Diabetic ketoacidosis, Lactic acidosis<br />
– Salicylate poison<strong>in</strong>g (children)<br />
– Methanol, ethylene glycol poison<strong>in</strong>g<br />
– Renal failure<br />
– Diarrhea<br />
• Metabolic Alkalosis<br />
– Prolonged vomit<strong>in</strong>g<br />
– Diuretic therapy<br />
– Hyperadrenocortical disease<br />
– Exogenous base (antacids, bicarbonate IV, citrate toxicity after<br />
massive blood transfusions)<br />
Metabolic Acidosis
Metabolic Alkalosis<br />
Respiratory Acidosis
Respiratory Alkalosis<br />
Electrolyte Balance<br />
Electrolytes have 4 important physiological functions <strong>in</strong> the <strong>body</strong><br />
• essential m<strong>in</strong>erals <strong>in</strong> certa<strong>in</strong> biochemical reactions<br />
• control osmosis = control the movement of water between<br />
compartments<br />
• ma<strong>in</strong>ta<strong>in</strong> acid-base <strong>balance</strong><br />
• conduct electrical currents (depolarization events)<br />
Regulators:<br />
Aldosterone ↑ [Na + ] [Cl - ] [H 2 O] ↓ [K + ]<br />
Atrial Natriuretic Peptide (opposite effect)<br />
Antidiuretic Hormone ↑ [H 2 O] (↓ [solutes])<br />
Parathyroid Hormone ↑ [Ca ++ ] ↓ [HPO 4- ]<br />
Calciton<strong>in</strong> (opposite effect)<br />
Female sex hormones ↑ [H 2 O]
Electrolytes<br />
• Sodium (Na + ) - 136-146 mmol/liter<br />
– Most abundant cation<br />
• major ECF cation (90% of cations present)<br />
• determ<strong>in</strong>es osmolarity of ECF<br />
Plasma OSM = 2[Na + ] + 2[K + ] + [Urea] + [Glucose]<br />
– Regulation<br />
• Aldosterone<br />
• ADH<br />
• ANP<br />
– Homeostatic im<strong>balance</strong>s<br />
• Hyponatremia<br />
• Hypernatremia<br />
Hypertonic Alterations - Related to sodium ga<strong>in</strong> or water loss<br />
• Hypernatremia<br />
– Serum sodium >146 mmol/L<br />
– Intake of hypertonic salt solution<br />
– Water movement from the ICF to the ECF<br />
• Intracellular dehydration<br />
– Manifestations: <strong>in</strong> consequence of cell dehydration<br />
• Excitability, convulsions, or on the other h<strong>and</strong> drows<strong>in</strong>ess<br />
accompany<strong>in</strong>g with pulmonary edema, tachycardia, etc.<br />
• Water deficit<br />
- Dehydration (osmotic diuresis at glycosuria (diabetes),<br />
gastro<strong>in</strong>test<strong>in</strong>al losses-osmotic diarrhea, <strong>in</strong>fectious enteritis, high<br />
fever, burn <strong>in</strong>jury, elevated perspiration)<br />
- Renal free water clearance<br />
- Manifestations:<br />
– Tachycardia, weak pulses<br />
– Elevated hematocrit <strong>and</strong> serum sodium level
Hypernatremia<br />
Serum sodium >160 mmol/L <strong>in</strong> 60 % lethal<br />
Therapy:<br />
at water deficit – isotonic solutions (physiological sal<strong>in</strong>e<br />
solution) or slightly hypotonic (2/3 F)<br />
at normovolemia or hypervolemia – thiazide diuretics<br />
(hydrochlorothiazide, decreas<strong>in</strong>g of <strong>electrolyte</strong>s reabsorption <strong>in</strong><br />
renal tubules) <strong>and</strong> 5% glucose<br />
frequent monitor<strong>in</strong>g of <strong>electrolyte</strong> plasma level dur<strong>in</strong>g the<br />
treatment, avoid to fast reestablishment of the <strong>electrolyte</strong> level<br />
(max 1-2 mmol/L/h <strong>and</strong> 12 mmol/L/day<br />
Hypotonic Alterations - Related to Hyponatremia or free water excess<br />
• Hyponatremia<br />
- Serum sodium level
Hyponatremia<br />
!<br />
- water moves <strong>in</strong>to cells based on osmolarity difference, bra<strong>in</strong> cells (neurons)<br />
decrease water uptake by compensatory mechanisms decreas<strong>in</strong>g the <strong>in</strong>tracellular<br />
osmolarity by elevation of K + efflux (dur<strong>in</strong>g 24h), <strong>and</strong> organic substances metabolism<br />
(dur<strong>in</strong>g 48h, e.g. elim<strong>in</strong>ation of polyalcohols, am<strong>in</strong>oacids, chol<strong>in</strong> derivates)<br />
!<br />
Therapy:<br />
the major risk is to fast reestablish the normal level of Na + ions. The lower<br />
osmolarity of neurons due to compensatory mechanisms causes water efflux from<br />
the neurons, the neurons will shr<strong>in</strong>k <strong>and</strong> released from myel<strong>in</strong> sheath!!!<br />
1. if the disnatremia was developed dur<strong>in</strong>g the last 48hrs, than fast correction<br />
could be made (1-2mmol/L/h)<br />
2. if plasma [Na + ] is 105-120 mmol/L <strong>and</strong> neurological symptoms are present make<br />
the correction by 1-2mmol/L/h<br />
without neurological symptoms the speed of correction could be only<br />
0.5mmol/L/h<br />
3. if plasma [Na + ] is less than 105 mmol/L, first 20 mmol/L at 1-2mmol/L/h, <strong>and</strong> then<br />
slowly<br />
Hyponatremia<br />
Calculation of total need of Na +<br />
mmol Na + = mass (kg) x f x (targeted Na + - determ<strong>in</strong>ed Na + )<br />
f = 0.6 for man<br />
f = 0.55 for woman<br />
Example: 70 kg weighted man has plasma [Na + ] 115 mmol/L, we would like to<br />
<strong>in</strong>crease the [Na + ] to 127 mmol/L/day (i.e. by 12 mmol/L)<br />
mmol Na + = 70 x 0.6 x (12) = 504<br />
concentrations of available salt solutions:<br />
0.9% NaCl (physiological sal<strong>in</strong>e solution)…1ml = 0.15 mmol Na + <strong>and</strong> Cl -<br />
10% NaCl ….1ml = 1.7 mmol Na + <strong>and</strong> Cl -<br />
5.8% NaCl …1ml = 1 mmol Na + <strong>and</strong> Cl -<br />
4.2% NaHCO 3<br />
….1ml = 0.5 mmol Na + <strong>and</strong> HCO 3<br />
-
• Chloride (Cl - ) - 95-108 mmol/L<br />
– Major ECF anion<br />
• helps <strong>balance</strong> osmotic potential <strong>and</strong> electrostatic equilibrium<br />
between fluid compartments<br />
• plasma membranes tend to be leaky to Cl - anions<br />
– Regulation: aldosterone<br />
– Homeostatic im<strong>balance</strong>s<br />
• Hypochloremia - results <strong>in</strong> muscle spasms, coma (usually<br />
occurs with hyponatremia) often due to prolonged vomit<strong>in</strong>g<br />
(elevated sweat chloride diagnostic of Cystic Fibrosis)<br />
Hypochloremia with normonatremia results <strong>in</strong> metabolic<br />
hypochloremic alkalosis<br />
Heperchloremia with normonatremia results <strong>in</strong> metabolic<br />
hyperchloremic acidosis<br />
Comb<strong>in</strong>ed dis<strong>balance</strong>s are treated based on the plasma [Na + ]<br />
Hypochloremia with normonatremia or hypernatremia (e.g. due to<br />
adm<strong>in</strong>istration of drugs with Na + , such as NaHCO 3<br />
, Na-lactate, Na-acetate,<br />
..), vomit<strong>in</strong>g at hyperaldosteronism (e.g. at activation of JG cells due to<br />
stenosis of renal artery)<br />
1. treatment of the cause, e.g. antiemetics at vomit<strong>in</strong>g<br />
2. solution NaCl, KCl at hypokalemia, 4.2% Arg<strong>in</strong><strong>in</strong>hydrochloride at significant<br />
alkalosis<br />
Calculation of total need of Cl -<br />
mmol Cl - = mass (kg) x 0.3 x BE<br />
where BE is base excess…. is <strong>in</strong> the normal range from -2.5 to +2.5 mmol/L, is equal to<br />
amount of strong acid (or base) which is needed to titrate 1L of plasma to pH 7.4 at normal<br />
pCO 2 (5.3 kPa <strong>and</strong> temp. 37 °C)<br />
<strong>in</strong> the case, where respiratory compensatory mechanism is <strong>in</strong>volved <strong>in</strong> regulation of pH (at<br />
the alkalosis is pCO 2 due to hypoventilation), then use Nejedly’s formula:<br />
mmol Cl - = mass (kg) x 0.3 x BE x<br />
pH determ<strong>in</strong>ed<br />
-pH targeted<br />
pH determ<strong>in</strong>ed<br />
-pH X<br />
7.248<br />
pCO 2<br />
2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5<br />
pH X<br />
7.67 7.61 7.567 7.517 7.487 7.457 7.427 7.397 7.377 7.35 7.332 7.314 7.298 7.28 7.263
Hyperchloremia with normonatreia<br />
1. treatment of the cause, obstruction of ur<strong>in</strong>ary tract (accompanied with<br />
hypernatremia <strong>and</strong> hyperkalemia), hypoaldosteronism, drug<br />
adm<strong>in</strong>istration e.g. HCl, NH4Cl, lys<strong>in</strong>e-HCl, arg<strong>in</strong><strong>in</strong>e-HCl), acute<br />
diarrhea (accompanied with hypokalemia)<br />
2. treatment of acidosis by NaHCO 3<br />
if pH of artery blood is
• Hypokalemia<br />
- Potassium level
Calcium (Ca 2+ )<br />
– Most abundant ion <strong>in</strong> <strong>body</strong><br />
• plasma 2.3-2.6 mmol/L<br />
• most stored <strong>in</strong> bone (98%) as hydroxyapatite<br />
- Necessary for structure of bones <strong>and</strong> teeth, blood clott<strong>in</strong>g, hormone<br />
secretion, <strong>and</strong> cell receptor function<br />
- Regulation:<br />
• Parathyroid Hormone (PTH) - ↑ blood Ca 2+<br />
• Calciton<strong>in</strong> (CT) - ↓ blood Ca 2+<br />
- Homeostatic im<strong>balance</strong>s:<br />
Hypocalcemia (necrotic pancreatitis, malabsorption, hypoparathyreosis,<br />
vitam<strong>in</strong> D deficit (osteomalacia)) - muscle cramps, convulsions<br />
Therapy: 1. cause, 2. 10% Ca-gluconicum (10ml ampules, 1ml =<br />
0.25mmol)<br />
Hypercalcemia (hyperparathyreosis, hypervitam<strong>in</strong>osis D, osteolytic tumor<br />
metastasis)- vomit<strong>in</strong>g, cardiovascular symptoms, coma (critical [Ca+] is<br />
above 3.75 mmol/l); prolonged abnormal calcium deposition, e.g.,<br />
stone formation<br />
Therapy: 1.cause, 2. <strong>in</strong>crease of diuresis by furosemide (at 3L/day),<br />
glucocorticoids decreas<strong>in</strong>g Ca+ absorption by <strong>in</strong>test<strong>in</strong>e, calciton<strong>in</strong><br />
Phosphate (H 2 PO 4- , HPO 4<br />
2-<br />
, PO 4<br />
3-<br />
)<br />
– Important ICF anions; plasma 0.7-1.5 mmol/L<br />
• most (85%) is stored <strong>in</strong> bone as calcium salts<br />
• also comb<strong>in</strong>ed with lipids, prote<strong>in</strong>s, carbohydrates, nucleic acids<br />
(DNA <strong>and</strong> RNA), <strong>and</strong> high energy phosphate transport compound<br />
• important acid-base buffer <strong>in</strong> <strong>body</strong> fluids<br />
– Regulation - regulated <strong>in</strong> an <strong>in</strong>verse relationship with Ca 2+ by PTH <strong>and</strong><br />
calciton<strong>in</strong> <strong>and</strong> Vitam<strong>in</strong> D (If the concentration of one <strong>in</strong>creases, that of<br />
the other decreases)<br />
– Parathyroid hormone (PTH) - Increases plasma calcium levels<br />
– Vitam<strong>in</strong> D (fat-soluble steroid) - Increases calcium absorption from the<br />
GI tract<br />
– Calciton<strong>in</strong> - Decreases plasma calcium levels<br />
– Homeostatic im<strong>balance</strong>s<br />
• Phosphate concentrations shift oppositely from calcium<br />
concentrations <strong>and</strong> symptoms are usually due to the related<br />
calcium excess or deficit
Hypophosphatemia <strong>and</strong> Hyperphosphatemia<br />
• Hypophosphatemia<br />
(abrosia, malnutrition, renal losses, hyperparathyreosis)<br />
– Osteomalacia (soft bones)<br />
– Muscle weakness<br />
– Bleed<strong>in</strong>g disorders (platelet impairment)<br />
– Anemia<br />
– Leukocyte alterations<br />
Therapy: significant decrease
Acid-Base Balance<br />
• Normal metabolism produces H + (acidity)<br />
• Three Homeostatic mechanisms:<br />
– Buffer systems - <strong>in</strong>stantaneous; temporary<br />
– Exhalation of CO 2 - operates with<strong>in</strong> m<strong>in</strong>utes; cannot<br />
completely correct serious im<strong>balance</strong>s<br />
– Kidney excretion - can completely correct any im<strong>balance</strong><br />
(eventually)<br />
• Buffer Systems<br />
– Consists of a weak acid <strong>and</strong> the salt of that acid which<br />
functions as a weak base<br />
Acid-Base Balance<br />
• Carbonic Acid - Bicarbonate Buffer<br />
– A weak base (carbonic anhydrase)<br />
H + + HCO 3- ⇔ H 2 CO 3 ⇔ H 2 O + CO 2<br />
• Phosphate Buffer<br />
NaOH + NaH 2 PO 4 ⇔ H 2 O + Na 2 HPO 4<br />
HCl + Na 2 HPO 4 ⇔ NaCl + NaH 2 PO 4<br />
- ma<strong>in</strong>ly <strong>in</strong>tracellularly, dur<strong>in</strong>g acidemia proton is bound, dur<strong>in</strong>g alkalemia proton is<br />
released via cell membrane K + /H + antiporter<br />
• Prote<strong>in</strong> Buffer (resp. album<strong>in</strong> & hemoglob<strong>in</strong> )<br />
Am<strong>in</strong>o acids have am<strong>in</strong>e group (proton<br />
acceptor = weak base) <strong>and</strong> a carboxyl group<br />
(proton donor = weak acid)<br />
hemoglob<strong>in</strong>- oxyhemoglob<strong>in</strong> systém, where<br />
oxyhemoglob<strong>in</strong> is stronger acid than<br />
hemoglob<strong>in</strong> (proton is more simply released)
Acid-Base <strong>balance</strong> systems <strong>in</strong> blood<br />
1. Carbonic Acid - Bicarbonate Buffer 53 %<br />
2. Hemoglob<strong>in</strong>-oxyhemoglob<strong>in</strong> 35 %<br />
3. Plasma prote<strong>in</strong>s 7 %<br />
4. Phosphate buffers 5 %<br />
plasma pH 7.37-7.43<br />
Examples:<br />
Prescribe <strong>in</strong>fusion therapy for m<strong>in</strong>eral blood alteration:<br />
a) 70 kg man with polyuria 3.5 L/d, plasma [K + ] = 2.8 mmol/L, ur<strong>in</strong>e<br />
[K + ] = 13 mmol/L <strong>and</strong> with normal levels of other m<strong>in</strong>erals <strong>and</strong><br />
normal blood pH<br />
b) 60 kg woman with plasma [Cl - ] = 78 mmol/L with normal levels of<br />
other m<strong>in</strong>erals <strong>and</strong> blood pH 7.52, pCO 2 6.5 kPa, BE +4.2 <strong>and</strong><br />
normal renal function