Using JCP format - Université de Mons
Using JCP format - Université de Mons
Using JCP format - Université de Mons
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
8562 J. Chem. Phys., Vol. 112, No. 19, 15 May 2000 Coheur et al.<br />
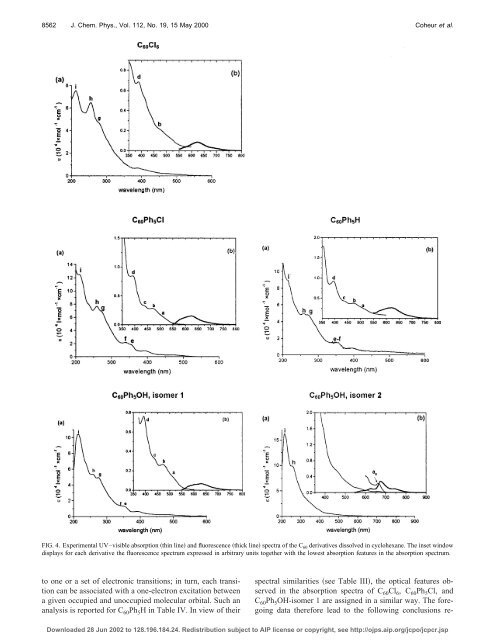
FIG. 4. Experimental UV–visible absorption thin line and fluorescence thick line spectra of the C 60 <strong>de</strong>rivatives dissolved in cyclohexane. The inset window<br />
displays for each <strong>de</strong>rivative the fluorescence spectrum expressed in arbitrary units together with the lowest absorption features in the absorption spectrum.<br />
to one or a set of electronic transitions; in turn, each transition<br />
can be associated with a one-electron excitation between<br />
a given occupied and unoccupied molecular orbital. Such an<br />
analysis is reported for C 60 Ph 5 H in Table IV. In view of their<br />
spectral similarities see Table III, the optical features observed<br />
in the absorption spectra of C 60 Cl 6 , C 60 Ph 5 Cl, and<br />
C 60 Ph 5 OH-isomer 1 are assigned in a similar way. The foregoing<br />
data therefore lead to the following conclusions re-<br />
Downloa<strong>de</strong>d 28 Jun 2002 to 128.196.184.24. Redistribution subject to AIP license or copyright, see http://ojps.aip.org/jcpo/jcpcr.jsp