2010 Chapter 8 homework SOLUTIONS _v2 - Department of ...
2010 Chapter 8 homework SOLUTIONS _v2 - Department of ...
2010 Chapter 8 homework SOLUTIONS _v2 - Department of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Read Section 8.6 (Acid/Base chemistry) and do exercises 8-11 on pages 300-304<br />
and problems 38, 46, and 47 on page 328-329.<br />
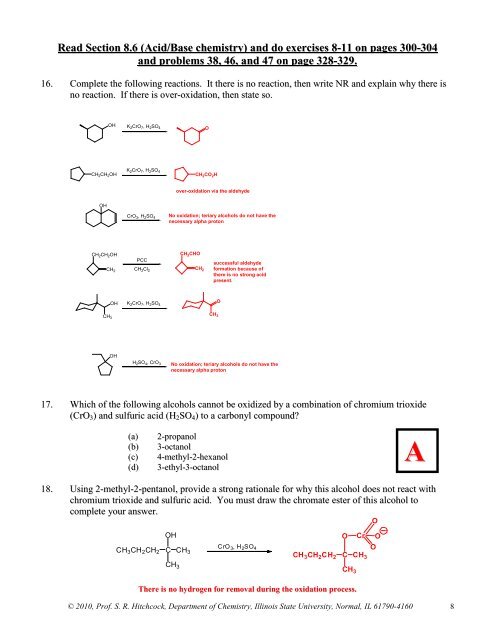
16. Complete the following reactions. It there is no reaction, then write NR and explain why there is<br />
no reaction. If there is over-oxidation, then state so.<br />
OH<br />
K 2 CrO 7 , H 2 SO 4<br />
O<br />
CH 2 CH 2 OH<br />
K 2 CrO 7 , H 2 SO 4<br />
CH 2 CO 2 H<br />
over-oxidation via the aldehyde<br />
OH<br />
CrO 3 , H 2 SO 4<br />
No oxidation; teriary alcohols do not have the<br />
necessary alpha proton<br />
CH 2 CH 2 OH<br />
CH 2<br />
PCC<br />
CH 2 Cl 2<br />
OH K 2 CrO 7 , H 2 SO 4<br />
CH 3<br />
CH 2 CHO<br />
CH 2<br />
successful aldehyde<br />
formation because <strong>of</strong><br />
there is no strong acid<br />
present.<br />
CH 3<br />
O<br />
OH<br />
H 2 SO 4 , CrO 3<br />
No oxidation; teriary alcohols do not have the<br />
necessary alpha proton<br />
17. Which <strong>of</strong> the following alcohols cannot be oxidized by a combination <strong>of</strong> chromium trioxide<br />
(CrO 3 ) and sulfuric acid (H 2 SO 4 ) to a carbonyl compound<br />
(a)<br />
(b)<br />
(c)<br />
(d)<br />
2-propanol<br />
3-octanol<br />
4-methyl-2-hexanol<br />
3-ethyl-3-octanol<br />
A<br />
18. Using 2-methyl-2-pentanol, provide a strong rationale for why this alcohol does not react with<br />
chromium trioxide and sulfuric acid. You must draw the chromate ester <strong>of</strong> this alcohol to<br />
complete your answer.<br />
CH 3 CH 2 CH 2<br />
OH<br />
O Cr O<br />
CrO<br />
C CH 3 , H 2 SO 4 O<br />
3<br />
CH 3 CH 2 CH 2 C CH 3<br />
CH 3<br />
CH 3<br />
There is no hydrogen for removal during the oxidation process.<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 8<br />
O