2010 Chapter 8 homework SOLUTIONS _v2 - Department of ...
2010 Chapter 8 homework SOLUTIONS _v2 - Department of ...
2010 Chapter 8 homework SOLUTIONS _v2 - Department of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ILLINOIS STATE UNIVERSITY<br />
DEPARTMENT OF CHEMISTRY, ILLINOIS STATE UNIVERSITY, NORMAL, IL 61790-4160<br />
<strong>Chapter</strong> 8: The Chemistry <strong>of</strong> the Alcohols<br />
Read Section 8.1 (Nomenclature) and do exercises 8.1-8.2 on page 289 and<br />
problems 24 and 25 and page 326.<br />
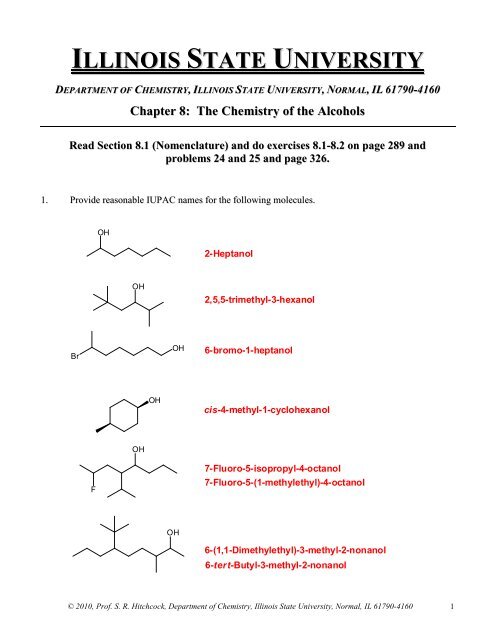
1. Provide reasonable IUPAC names for the following molecules.<br />
OH<br />
2-Heptanol<br />
OH<br />
2,5,5-trimethyl-3-hexanol<br />
Br<br />
OH<br />
6-bromo-1-heptanol<br />
OH<br />
cis-4-methyl-1-cyclohexanol<br />
OH<br />
F<br />
7-Fluoro-5-isopropyl-4-octanol<br />
7-Fluoro-5-(1-methylethyl)-4-octanol<br />
OH<br />
6-(1,1-Dimethylethyl)-3-methyl-2-nonanol<br />
6-tert-Butyl-3-methyl-2-nonanol<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 1
2. Provide acceptable IUPAC names for the following molecules.<br />
A<br />
OH<br />
B OH<br />
Br<br />
A. 7-Bromo-4-(1-methylethyl)-2-octanol or 7-Bromo-4-isopropyl-2-octanol<br />
B. trans-2-(1,1-dimethylethyl)cyclopentane or trans-2-tert-butylcyclopentane<br />
3. Provide acceptable IUPAC names for the following molecules.<br />
4. Draw a reasonable structure for the molecule (S)-3-methyl-3-heptanol.<br />
OH<br />
CH 3 CH 2 CH 2 CH 2<br />
C CH 2 CH 3<br />
CH 3<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 2
Read Section 8.2 (Properties) and do problems 26 and 27 page 326.<br />
5. The boiling point <strong>of</strong> methane is -161.7 o C and the boiling point <strong>of</strong> methanol is 65 o C. Provide a<br />
strong defense for why there is greater than 200 o C difference in temperatures.<br />
This involves the intermolecular forces that hold collections <strong>of</strong> these molecules together. The<br />
bonds in methane are not polarized and so there is virtually no attraction between these<br />
molecules. The OH group in methanol is strongly polarized positive at the hydrogen and<br />
negative at the oxygen. This leads to extensive intermolecular bonding which gives rise to<br />
the higher temperature requirement to break up this interaction.<br />
6. Provide a strong rationale for why 1,2-ethanediol has a boiling point <strong>of</strong> 196-198 o C and butane has<br />
a boiling point <strong>of</strong> -0.5 o C. Why are these two compounds so different<br />
The molecular weights for these compounds are nearly the same. In the case <strong>of</strong> butane,<br />
intermolecular London forces are responsible for the low boiling point. The instantaneous<br />
dipole-dipole attraction is weak and not sufficient enough to give rise to a higher boiling<br />
point. The compound 1,2-ethanediol has significant hydrogen bonding that serves to create<br />
an intermolecular network <strong>of</strong> strong hydrogen bonding between molecules.<br />
7. Consider the general structure for the α-amino acids: RCH(NH 2 )CO 2 H. Which <strong>of</strong> the side chains<br />
below would enhance the hydrophilicity <strong>of</strong> an α-amino acid<br />
(a) R = -CH 2 Ph<br />
(b) R = -CH 2 OH<br />
(c) R = -CH 2 (CH 2 ) 16 CH 3<br />
(d) R = -CH(CH 3 ) 2<br />
B<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 3
Read Section 8.3 (Acid/Base chemistry) and do exercises 4-6 on pages 293-294 and<br />
problems 30 and 31 on page 327.<br />
8. Write out the dissociation expression for 1-butanol in water and the corresponding K a expression.<br />
What is the average pK a for most alcohols<br />
The average pK a is about 16 to 19.<br />
CH 3 CH 2 CH 2 CH 2 OH + H 2 O CH 3 CH 2 CH 2 CH 2 O + H 3 O<br />
CH 3 CH 2 CH 2 CH 2 O<br />
H 3 O<br />
K a =<br />
CH 3 CH 2 CH 2 CH 2 OH<br />
9. Which <strong>of</strong> the following acids has the greater acidity, CH 3 CH 2 OH or F 3 CCH 2 OH Provide a<br />
strong rationale for your answer. It is not sufficient to suggest that one material has a lower pK a<br />
than the other.<br />
F 3 CCH 2 OH has the greater acidity due to the presence <strong>of</strong> the electronegative fluorines.<br />
These fluorines stabilize the buildup <strong>of</strong> negative charge on the oxygen. A more stable<br />
conjugate base leads to a more acidic compound. See page 292 in the text.<br />
10. Which one <strong>of</strong> the following reagents would not be effective in terms <strong>of</strong> completely removing an<br />
alcoholic proton (RO-H) from an alcohol such as butanol<br />
(a) Na + - OH (sodium hydroxide)<br />
(b) Na + - NH 2 (sodium amide)<br />
A<br />
(c) K + - H (potassium hydride)<br />
(d) Li + - CH 2 CH 2 CH 2 CH 3 (n-butyllithium)<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 4
Read Section 8.5 (Acid/Base chemistry) and do exercise 7 on pages 296 and<br />
problem 34 on page 327.<br />
11. Complete the following reactions. If there is no reaction, then write NR.<br />
Br<br />
KOH, dmf<br />
S N 2<br />
OH<br />
Cl<br />
OH<br />
H<br />
KOH, dmf<br />
S N 2-E2 mix<br />
H<br />
H<br />
I<br />
Br<br />
H 2 O<br />
S N 1-E1<br />
1. O S N 2<br />
H 3 C O<br />
Na<br />
H<br />
H<br />
OH<br />
O<br />
O CH 3<br />
H<br />
H<br />
OH<br />
H<br />
H<br />
OH<br />
2. NaOH<br />
OMs<br />
1.<br />
O<br />
O<br />
Na<br />
S N 2<br />
O<br />
O CH 3<br />
OH<br />
2. KOH<br />
Cl<br />
H 2 O<br />
S N 1-E1<br />
OH<br />
Cl<br />
NaOH, 0 o C<br />
E2<br />
12. Write in the reagent that would most effectively convert the following alkyl halides into their<br />
corresponding alcohols. No stereochemistry is implied for these reactions.<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 5
Read Section 8.6 (Acid/Base chemistry) and do exercises 8-11 on pages 300-304<br />
and problems 38, 46, and 47 on page 328-329.<br />
13. Complete the following reactions concerning the reduction <strong>of</strong> carbonyl compounds. If there is no<br />
reaction, then write NR.<br />
O<br />
OH<br />
H<br />
NaBH 4 , CH 3 OH<br />
H<br />
H<br />
O<br />
OH<br />
NaBH 4 , CH 3 OH<br />
H<br />
O<br />
H<br />
OH<br />
1. LiAlH 4 , THF<br />
2. dilute HCl<br />
O<br />
1. LiAlH 4 , THF<br />
2. dilute HCl<br />
OH<br />
H<br />
O<br />
NaBH 4 , CH 3 OH<br />
OH<br />
H<br />
O<br />
1. LiAlH 4 , THF<br />
2. dilute HCl<br />
OH<br />
H<br />
O<br />
1. LiAlH 4 , THF<br />
2. dilute HCl<br />
H<br />
H<br />
OH<br />
14. Which <strong>of</strong> the reagents below would you use to accomplish the following transformation<br />
(a)<br />
(b)<br />
(c)<br />
(d)<br />
O<br />
HO<br />
<br />
D<br />
D = deuterium ( 2 H)<br />
LiAlD 4 in ether followed by treatment with H 2 O<br />
LiAlD 4 in ether followed by treatment with D 2 O<br />
LiAlH 4 in ether followed by treatment with H 2 O<br />
LiAlH 4 in ether followed by treatment with D 2 O<br />
A<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 6
Read Section 8.6 (Acid/Base chemistry) and do exercises 8-11 on pages 300-304<br />
and problems 38, 46, and 47 on page 328-329.<br />
15. Complete the following reactions concerning the reduction <strong>of</strong> carbonyl compounds.<br />
CH 2 CHO<br />
NaBH 4 , CH 3 OH<br />
CH 2 CH 2 OH<br />
O<br />
H<br />
OH<br />
NaBH 4 , CH 3 OH<br />
CH 3 O<br />
O<br />
CH 3 O<br />
OH<br />
1. LiAlH 4 , THF<br />
2. dilute HCl<br />
H<br />
O<br />
O<br />
H<br />
OH<br />
OH<br />
H<br />
H CH 3<br />
1. LiAlH 4 , THF<br />
H CH 3<br />
2. dilute HCl<br />
CHO<br />
1. LiAlH 4 , THF<br />
CH 2 OH<br />
2. dilute HCl<br />
O<br />
1. LiAlH 4 , THF<br />
H<br />
OH<br />
2. dilute HCl<br />
O<br />
HO<br />
H<br />
NaBH 4 , CH 3 OH<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 7
Read Section 8.6 (Acid/Base chemistry) and do exercises 8-11 on pages 300-304<br />
and problems 38, 46, and 47 on page 328-329.<br />
16. Complete the following reactions. It there is no reaction, then write NR and explain why there is<br />
no reaction. If there is over-oxidation, then state so.<br />
OH<br />
K 2 CrO 7 , H 2 SO 4<br />
O<br />
CH 2 CH 2 OH<br />
K 2 CrO 7 , H 2 SO 4<br />
CH 2 CO 2 H<br />
over-oxidation via the aldehyde<br />
OH<br />
CrO 3 , H 2 SO 4<br />
No oxidation; teriary alcohols do not have the<br />
necessary alpha proton<br />
CH 2 CH 2 OH<br />
CH 2<br />
PCC<br />
CH 2 Cl 2<br />
OH K 2 CrO 7 , H 2 SO 4<br />
CH 3<br />
CH 2 CHO<br />
CH 2<br />
successful aldehyde<br />
formation because <strong>of</strong><br />
there is no strong acid<br />
present.<br />
CH 3<br />
O<br />
OH<br />
H 2 SO 4 , CrO 3<br />
No oxidation; teriary alcohols do not have the<br />
necessary alpha proton<br />
17. Which <strong>of</strong> the following alcohols cannot be oxidized by a combination <strong>of</strong> chromium trioxide<br />
(CrO 3 ) and sulfuric acid (H 2 SO 4 ) to a carbonyl compound<br />
(a)<br />
(b)<br />
(c)<br />
(d)<br />
2-propanol<br />
3-octanol<br />
4-methyl-2-hexanol<br />
3-ethyl-3-octanol<br />
A<br />
18. Using 2-methyl-2-pentanol, provide a strong rationale for why this alcohol does not react with<br />
chromium trioxide and sulfuric acid. You must draw the chromate ester <strong>of</strong> this alcohol to<br />
complete your answer.<br />
CH 3 CH 2 CH 2<br />
OH<br />
O Cr O<br />
CrO<br />
C CH 3 , H 2 SO 4 O<br />
3<br />
CH 3 CH 2 CH 2 C CH 3<br />
CH 3<br />
CH 3<br />
There is no hydrogen for removal during the oxidation process.<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 8<br />
O
Read Section 8.7 (organometallics chemistry) and do exercise 13 on pages 307-309<br />
and problem 40 on page 328.<br />
19. Complete the following reactions and identify the carbon atom that carries the negative charge.<br />
Cl<br />
2 Li metal, ether<br />
Cl<br />
+ LiCl<br />
H 3 C<br />
Br<br />
OCH 3<br />
Magnesium metal<br />
ether<br />
H 3 C<br />
MgBr<br />
OCH 3<br />
Cl<br />
2 Li metal, ether<br />
Li<br />
+ LiCl<br />
CH 2 Cl<br />
Magnesium metal<br />
ether<br />
CH 2 MgCl<br />
Br<br />
2 Li metal, ether<br />
Li<br />
+ LiBr<br />
CH 2 F<br />
Magnesium metal<br />
ether<br />
No reaction. The carbon-fluorine bond is<br />
too strong.<br />
CH 3<br />
2 Li metal, ether<br />
No reaction. There is no halogen<br />
leaving group.<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 9
20. Indicate the polarization <strong>of</strong> each <strong>of</strong> the BOLD bonds in the following molecules. The first<br />
structure is completed for you.<br />
δ<br />
δ<br />
H<br />
H<br />
δ<br />
Cl<br />
δ<br />
Br<br />
hydrogen chloride<br />
hydrogen chloride<br />
δ<br />
δ<br />
H O H<br />
water<br />
H<br />
H<br />
C<br />
H<br />
δ<br />
O<br />
H<br />
δ<br />
(methanol, CH 3 OH)<br />
H 3 C<br />
O<br />
H<br />
O<br />
δ<br />
δ<br />
acetic acid, a carboxylic acid<br />
sometimes written as CH 3 CO 2 H<br />
O<br />
H<br />
O<br />
δ<br />
δ<br />
benzoic acid, a carboxylic acid<br />
21. The following reactions involve quenching organometallic reagents. These reactions are driven by<br />
the formation <strong>of</strong> more stable compounds.<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 10
Read Section 8.8 (organometallics chemistry) and do exercises 15 and 16 on page 309<br />
and problems 43, 44, and 53 on pages 328-329.<br />
22. Complete the following reactions. If there is no reaction, then write NR.<br />
H<br />
O<br />
H<br />
1.<br />
2. dilute HCl<br />
MgBr<br />
OH<br />
H<br />
H<br />
O<br />
OH<br />
H<br />
1. CH 3 CH 2 MgBr<br />
2. dilute HCl<br />
H<br />
CH 2 CH 3<br />
O<br />
1. CH 3 CH 2 OCH 2 Li<br />
2. dilute HCl<br />
HO CH 2 OCH 2 CH 3<br />
O<br />
1.<br />
MgBr<br />
HO<br />
2. dilute HCl<br />
O<br />
1.<br />
CH 2 CH 2 Li<br />
HO<br />
2. dilute HCl<br />
CH 3<br />
O<br />
1.<br />
MgCl<br />
CH 2 CH 2<br />
OH<br />
CH 3<br />
2. dilute HCl<br />
23. What combination <strong>of</strong> reagents would NOT form the alcohol shown after a work up with dilute<br />
HCl Circle the best choice.<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 11
24. Complete the following reactions by writing in the products. If there is no reaction, then you<br />
should write, NR.<br />
Br<br />
Mg o , ether<br />
redox<br />
MgBr<br />
Cl<br />
CH 2 OMe<br />
Li o , ether<br />
redox<br />
Li<br />
CH 2 OMe<br />
+ LiCl<br />
Br<br />
O<br />
H<br />
1. CH 3 CH 2 MgBr<br />
2. dilute HCl<br />
Br<br />
OH<br />
H<br />
CH 2 CH 3<br />
O<br />
1.<br />
Li<br />
ether<br />
OH<br />
2. dilute HCl<br />
OCH 3<br />
CH 3<br />
1. Magnesium, ether<br />
OCH 3<br />
CH 3<br />
2.<br />
Br<br />
CH 3 O<br />
MgCl<br />
H 3 C<br />
CHO<br />
O<br />
OH<br />
CH 3 O<br />
HO<br />
H<br />
H<br />
O<br />
+<br />
H 3 C<br />
O<br />
MgCl<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 12
25. Complete the following. If there are multiple products, then you must clearly state which product<br />
is the major product. Assume a work up for all reactions.<br />
O<br />
O<br />
1. LiAlD 4 , ether<br />
2. dilute HCl<br />
NaBH 4 , CH 3 OH<br />
OH<br />
D<br />
OH<br />
H<br />
CH 3<br />
O<br />
1. LiAlH 4 , ether<br />
2. dilute HCl<br />
CH 3<br />
H<br />
OH<br />
O<br />
OH<br />
1. LiAlH 4 , ether<br />
2. dilute HCl<br />
CrO 3 , H 2 SO 4<br />
OH<br />
H<br />
O<br />
CH 3<br />
OH<br />
1.PCC, CH 2 Cl 2<br />
CH 3<br />
O<br />
CH 3<br />
OH<br />
H<br />
CH 3<br />
2. Cyclopropyl lithium<br />
H<br />
CH 3<br />
H<br />
CH 3<br />
O<br />
H<br />
OCH 3<br />
1. NaBH 4 , EtOH<br />
2. CD 3 MgBr<br />
OH<br />
H<br />
H<br />
OCH 3<br />
O<br />
H<br />
H<br />
OCH 3<br />
MgBr<br />
+ CD 3 H<br />
Na 2 Cr 2 O 7 , H 2 SO 4<br />
OH<br />
OH<br />
O<br />
O<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 13
26. Complete the following integrated reactions. Assume a work up for all reactions.<br />
Br<br />
1. Mg o , ether<br />
2. CH 3 CH 2 CHO<br />
MgBr<br />
OH<br />
C<br />
O<br />
CH 2 CH 3<br />
3. Na 2 Cr 2 O 7 , H 2 SO 4<br />
OCH 3<br />
H CH 2 CH 3 C<br />
OCH 3<br />
OCH 3<br />
(CH 3 ) 2 CH<br />
OCH 3<br />
OH<br />
OH<br />
C H<br />
H<br />
1.<br />
N<br />
H<br />
CrO 3 Cl<br />
(PCC)<br />
2. CH 3 CH 2 MgCl<br />
3. Na 2 Cr 2 O 7 , H 2 SO 4<br />
4. CD 3 CD 2 MgBr, ether<br />
(CH 3 ) 2 CH<br />
(CH 3 ) 2 CH<br />
O<br />
C H (CH 3 ) 2 CH<br />
OH<br />
C H<br />
CH 2 CH 3<br />
O<br />
C<br />
CH 2 CH 3<br />
OH<br />
(CH 3 ) 2 CH<br />
C<br />
CD 2 CD 3<br />
CH 2 CH 3<br />
O<br />
1. PCC, CH 2 Cl 2<br />
2. CD 3 CH 2 CH 2 MgBr<br />
3. dilute HCl<br />
H<br />
OH<br />
CH 2 CH 2 CD 3<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 14
27. Complete the following problems. If there is no reaction, then write NR.<br />
O<br />
OH<br />
CH 2 CH 3<br />
1.<br />
CH 3 CH 2 MgBr, THF<br />
2. dilute HCl<br />
CH 2 CH 3<br />
CH 2 CH 3<br />
OH<br />
O<br />
CH 3<br />
1.<br />
CD 3 MgBr, THF<br />
2. dilute HCl<br />
CH 3<br />
CD 3<br />
O<br />
CH 3<br />
1.<br />
MgBr<br />
2. dilute HCl<br />
OH<br />
CH 3<br />
HO CH 2 CH 2<br />
O<br />
1.<br />
CH 2 CH 2 MgBr, THF<br />
2. dilute HCl<br />
O<br />
1. CH 3 CH 2 CH 2 MgCl<br />
HO<br />
CH 2 CH 2 CH 3<br />
2. dilute HCl<br />
O<br />
1. CH 3 CH 2 CH 2 MgCl<br />
OH<br />
H<br />
CH 2 CH(CH 3 ) 2<br />
2. dilute HCl<br />
H<br />
CH 2 CH(CH 3 ) 2<br />
CH 2 CH 2 CH 3<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 15
28. Complete the following integrated problems. Write out the products <strong>of</strong> each transformation.<br />
OH<br />
1. PCC, CH 2 Cl 2<br />
2. CH 3 MgBr<br />
O<br />
OH<br />
3. dilute HCl<br />
CH 3<br />
OH<br />
1. PCC, CH 2 Cl 2<br />
2. CH 3 CD 2 MgBr<br />
3. dilute HCl<br />
O<br />
OH<br />
CD 2 CH 3<br />
1. Na 2 Cr 2 O 7 , H 2 SO 4<br />
HO<br />
CH 3<br />
2.<br />
MgBr<br />
O<br />
CH 3<br />
HO<br />
CH 3<br />
3. dilute HCl<br />
OH<br />
1. PCC, CH 2 Cl 2<br />
O<br />
OH<br />
2. (CH 3 )CHCH 2 Li<br />
CH 2 CH(CH 3 ) 2<br />
3. dilute HCl<br />
OH<br />
1. K 2 Cr 2 O 7 , H 2 SO 4<br />
2. CH 3 MgBr<br />
3. dilute HCl<br />
No reaction. Tertiary alcohols cannot be<br />
oxidized because they do not have the<br />
hydrogen necessary for the elimination<br />
process.<br />
OH<br />
1. CrO 3 , H 2 SO 4<br />
2.<br />
Li<br />
O<br />
OH<br />
3. dilute HCl<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 16
Read Section 8.9 (organometallics chemistry) and do exercises 19, 20, and 21 on pages<br />
315-317 and problems 54-56 on pages 329-330.<br />
29. Complete the following problem. Show all possible products.<br />
1. PCC, CH 2 Cl 2<br />
2. CD 3 MgBr, ether (D = deuterium, 2 H)<br />
3. dilute HCl<br />
methanol<br />
4. PCC, CH 2 Cl 2<br />
5. CT 3 Li, ether (T = tritium, 3 H)<br />
6. dilute HCl<br />
7. PCC, CH 2 Cl 2<br />
8. CH 3 Na in ether<br />
9. dilute HCl<br />
H<br />
OH<br />
C H<br />
H<br />
O<br />
OH<br />
H C H H C H<br />
CD 3<br />
H<br />
O<br />
C<br />
CD 3<br />
H<br />
OH<br />
C CT 3<br />
CD 3<br />
O<br />
C CT 3<br />
CD 3<br />
H 3 C<br />
OH<br />
C<br />
CD 3<br />
CT 3<br />
Is this molecule chiral<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 17
30. Design at least two different syntheses for the molecule that is illustrated below.<br />
OH<br />
C CH 3<br />
CH 3<br />
OH<br />
OH<br />
C CH 3<br />
C CH 3<br />
CH 3<br />
CH 3<br />
OH<br />
C CH 3<br />
MgBr<br />
+<br />
O<br />
H 3 C CH 3<br />
CH 3<br />
O<br />
C CH 3<br />
+ CH 3 MgBr<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 18
31. Design at least three different syntheses for the molecule that is illustrated below.<br />
OH<br />
CH 3 CH 2<br />
C CH 3<br />
OH<br />
CH 3 CH 2<br />
OH<br />
C CH 3<br />
CH 3 CH 2<br />
C CH 3<br />
A<br />
C<br />
B<br />
CH 3 CH 2<br />
OH<br />
C CH 3<br />
OH<br />
CH 3 CH 2<br />
C CH 3<br />
OH<br />
O<br />
CH 3 CH 2<br />
C CH 3<br />
CH 3 CH 2<br />
C<br />
A<br />
CH 3 MgBr<br />
OH<br />
MgBr<br />
B<br />
CH 3 CH 2<br />
C CH 3<br />
O<br />
CH 3 CH 2<br />
C CH 3<br />
OH<br />
O<br />
C<br />
CH 3 CH 2<br />
C CH 3<br />
CH 3 CH 2 MgBr<br />
C CH 3<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 19
32. Complete the following retrosynthesis problems by writing in the appropriate reagents. No more<br />
than two steps are required for each <strong>of</strong> the following transformations.<br />
Cl<br />
Magnesium metal<br />
ether solvent<br />
MgCl<br />
Br<br />
OCH 3<br />
lithium metal<br />
ether solvent<br />
Li<br />
OCH 3<br />
Br<br />
1. Mg, ether<br />
2. dilute HCl<br />
H<br />
CH 2 Cl<br />
1. Li, ether<br />
2. O<br />
(<strong>Chapter</strong> 9)<br />
CH 2 CH 2 CH 2 OH<br />
O<br />
CH 3<br />
1. CH 3 CH 2 CH 2 MgBr<br />
OHCH 3<br />
2. dilute HCl<br />
CH 2 CH 2 CH 3<br />
© <strong>2010</strong>, Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> Chemistry, Illinois State University, Normal, IL 61790-4160 20