Quick Start with EC-Lab CYCLIC VOLTAMMETRY - CePoL/MC ...
Quick Start with EC-Lab CYCLIC VOLTAMMETRY - CePoL/MC ...
Quick Start with EC-Lab CYCLIC VOLTAMMETRY - CePoL/MC ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Quick</strong> <strong>Start</strong><br />
<strong>with</strong><br />
<strong>EC</strong>-<strong>Lab</strong> Ä<br />
<strong>CYCLIC</strong> <strong>VOLTAMMETRY</strong><br />
<strong>EC</strong>-<strong>Lab</strong> Ä Version 9.9<br />
1
<strong>Start</strong>ing a Cyclic Voltammetry <strong>with</strong> <strong>EC</strong>-<strong>Lab</strong> Ä<br />
This short tutorial goes through the states<br />
of selecting, setting up and running a typical Cyclic<br />
voltammetry experiment. The setting file (.mps),<br />
raw data file (.mpr) and processed file (.mpp)<br />
corresponding to this experiment are in the data<br />
directory.<br />
Cyclic voltammetry (CV) is the most widely used<br />
technique for acquiring qualitative information<br />
about electrochemical reactions. CV provides<br />
information on redox processes, heterogeneous<br />
electron-transfer reactions and adsorption<br />
processes. It offers a rapid location of redox<br />
potential of the electroactive species. CV consists<br />
of scanning linearly the potential of a stationary<br />
working electrode using a triangular potential<br />
waveform. During the potential sweep, the<br />
potentiostat measures the current resulting from<br />
electrochemical reactions (consecutive to the<br />
applied potential).<br />
The cyclic voltammogram is a current response as<br />
a function of the applied potential.<br />
The aim of this tutorial is to lead the user<br />
for the first time in <strong>EC</strong>-<strong>Lab</strong> Ä<br />
software. A Cyclic<br />
voltammetry protocol will be built and run to<br />
describe the instrument and the software. The<br />
operating conditions will be described in the<br />
experimental part (conditions just given as<br />
example). These experiments should not be<br />
considered as scientific results but just as example<br />
to start <strong>EC</strong>-<strong>Lab</strong> Ä<br />
software. This paper is not a<br />
scientific article.<br />
I- <strong>Start</strong>ing a Cyclic voltammetry experiment<br />
<strong>with</strong> <strong>EC</strong>-<strong>Lab</strong> Ä software<br />
When starting <strong>with</strong> <strong>EC</strong>-<strong>Lab</strong> Ä for the first time, the<br />
user has to report to the corresponding part in<br />
<strong>EC</strong>-<strong>Lab</strong> Ä software Help Menu. The user has to set<br />
his name and choose a channel on the global view<br />
(double click on the channel). On the setting<br />
window, click “Modify” and “New<br />
experiment”. The technique selection window<br />
appears.<br />
Fig.1: <strong>EC</strong>-<strong>Lab</strong> Ä technique window <strong>with</strong> a description of the protocol.<br />
1) In “Voltamperometric Techniques”, choose<br />
“Cyclic voltammetry” and click on “Ok”.<br />
One can see a picture and a description of this<br />
protocol on the right window. This protocol<br />
corresponds to one or several potential sweep<br />
between two potential limits.<br />
2) Then the setting window <strong>with</strong> Cyclic<br />
voltammetry protocol appears. Three different<br />
tabs are shown: “Advanced settings, Cell<br />
characteristics and Parameter settings”. The<br />
2
default setting window is “Parameter settings”.<br />
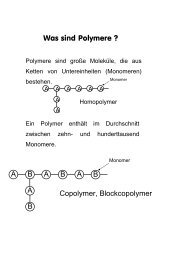
II- Cyclic voltammetry protocol description<br />
Let's consider the Parameter settings window <strong>with</strong><br />
Cyclic voltammetry detailed flow diagram. It is<br />
made <strong>with</strong> four different blocks (fig. 2). This<br />
protocol begins <strong>with</strong> an open circuit period (block<br />
1). No potential or current is applied to the<br />
electrochemical cell. Then the potential where the<br />
experiment will start (E i ) is defined (block 2) in<br />
absolute or according to the previous open circuit<br />
or measured or controlled potential. The sweep<br />
potential is scanned <strong>with</strong> a scan rate dE/dt from Ei<br />
to E 1 and E 2 (block 3). An average current is<br />
measured and recorded. The cyclic voltammetry<br />
experiment can be repeated n c times in order to<br />
have more than one cycle. The final potential of<br />
the experiment is defined (block 4).<br />
Block 1<br />
Block 2<br />
Block 3<br />
Block 4<br />
Fig. 2: Cyclic Voltammetry detailed flow diagram.<br />
II.1 Description of a CV experiment:<br />
Block 1: Open circuit potential sequence.<br />
Rest for t R = 1 s<br />
This block becomes colored when it is taken into<br />
account in the experiment. When its color was<br />
grey, the block was ignored.<br />
Note: This part of the experiment can be<br />
considered as a preconditioning period.<br />
If there are no parameters in the boxes of one<br />
block, the block is grey.<br />
Block 2: starting potential.<br />
Set E we to E i = 0 vs. E oc<br />
The experiment will begin at the open circuit<br />
potential. It is possible to define the initial potential<br />
as a function of the reference potential, the value<br />
of previously controlled or measured potential.<br />
3
Block 3: Potential sweep.<br />
Scan E we <strong>with</strong> dE/dt = 20 mV/s, (50 ÅV /<br />
26 ms)<br />
Notes:<br />
1) Scan rate setting<br />
If entering the potential scan rate in mV/s<br />
the default choice of the system proposes a<br />
scan rate, as close as possible to the<br />
requested one and obtained <strong>with</strong> the<br />
smallest possible step amplitude. To adjust<br />
the potential control resolution report to the<br />
technical note # 8.<br />
2) The scan rate is defined by dE/dt in<br />
absolute (it is not needed to set a negative<br />
value for a scan through lower potential<br />
values).<br />
to vertex potential E 1 = 0.7 V vs. Ref<br />
Definition of the potential limit of the reverse scan.<br />
reverse scan to vertex E 2 = -0.3 V vs. Ref<br />
windows are available in <strong>EC</strong>-<strong>Lab</strong> Ä<br />
depending on the experiment.<br />
software<br />
A particular window has been designed for battery<br />
electrode materials acting as intercalation<br />
electrode. For cyclic voltammetry and all other<br />
experiments the “Cell characteristics” window is<br />
used to:<br />
1) Describe the electrochemical cell (experimental<br />
conditions).<br />
2) Select the recording of the counter electrode<br />
potential.<br />
3) Select the recording of external signals ( pH, T,<br />
P,...) using auxiliary inputs 1, 2 and 3.<br />
4) Set the electrode surface area, characteristic<br />
mass of the material and the reference<br />
electrode used.<br />
Note: It is very important to set comments in<br />
maximum boxes in order to find the<br />
experimental conditions easily.<br />
Repeat n c = 2 times<br />
Measure over the last 50 % of the step<br />
duration<br />
Note:<br />
The user can select the part of the potential<br />
step for the current average ()<br />
calculation. It can be interesting to exclude<br />
the first points where the current may be<br />
perturbed by the step establishment.<br />
Record averaged over N = 1 voltage<br />
step(s)<br />
Once selected, an estimation of the number of<br />
points per cycle is displayed into the flow diagram.<br />
<strong>with</strong> I range = 100 ÅA bandwidth = 5-<br />
medium<br />
Note: The experiment will be made <strong>with</strong> three<br />
cycles (the first one is repeated two times).<br />
Block 4: final potential.<br />
End scan to Ef = 0 V vs. E oc<br />
The user can find and load the setting file of the<br />
experiment described on figure 2 in<br />
C:\<strong>EC</strong>-<strong>Lab</strong>\data\samples\CV_Fe_basique_1.mps.<br />
To do that click on “Load set” in the experiment<br />
frame and choose the file in the correct directory.<br />
II.3- Setting “Cell characteristics”<br />
The second window called “Cell characteristics”<br />
(fig. 3) is dedicated to the electrochemical cell<br />
parameters. Two different “Cell characteristics”<br />
Fig. 3: Cell characteristics window for the cyclic<br />
voltammetry protocol.<br />
III- Experimental part<br />
The system used in the CV experiment<br />
was a three electrodes system <strong>with</strong> an Au/Pd alloy<br />
as working electrode, a platinum wire as counter<br />
electrode and Saturated calomel electrode as<br />
reference electrode. The electrochemical cell<br />
contained a solution <strong>with</strong> NaCl (0.1 mol.L -1 ) as<br />
electrolyte and K 3 Fe(CN) 6 /K 4 Fe(CN) 6 equimolar<br />
(2.10 -3 mol.L -1 ). The pH of the solution was<br />
adjusted to 10.5 <strong>with</strong> NaOH (5 mol.L -1 ).<br />
The electrochemical conditions of the experiment<br />
can be found in<br />
4
C:\<strong>EC</strong>-<strong>Lab</strong>\data\samples\CV_Fe_1.mps.<br />
IV- Running a Cyclic voltammetry<br />
experiment.<br />
IV.1- Running the experiment on the<br />
standard testing box<br />
1) Click “Accept”. The following warning message<br />
will be displayed:<br />
“Warning: line 0 relaxation, there will be no<br />
recorded point (dE R =dt R =o)! Continue”<br />
This message says that no data point will be<br />
recorded during the OCV period.<br />
2) Click “Ok” and “Run” to start the experiment.<br />
The “Save As” window appears. The user has<br />
to define the file name and the directory to put<br />
the raw data file before the beginning of the<br />
experiment.<br />
3) Click on “Ok” and the graphic window is<br />
displayed. The data points are displayed<br />
automatically.<br />
4) On the graphic window one can see the<br />
graphic of figure 4.<br />
The operating conditions are described in the<br />
figure 1. At the beginning of the experiment, the<br />
cell was to the open circuit potential.<br />
Fig. 4: Cyclic voltammetry on the linear system of<br />
testing box 1 <strong>with</strong> the conditions defined before.<br />
The results of this experiment can be used to<br />
check that the instrument works fine and to be sure<br />
that the experiment has been well performed by<br />
the user. With the ohm law (U=R.I) it is very easy<br />
to compare the resistance value on the test box 1<br />
(5069 ohms) <strong>with</strong> the experimental value resulting<br />
from potential and current measurement for a<br />
given time (for example Ewe = -0.3 V and<br />
I = -0.059 mA). The current response follows the<br />
potential sweep.<br />
IV.2- Running an experiment in an<br />
electrochemical cell<br />
After the test box 1, the user can repeat the<br />
experiment in a real electrochemical cell <strong>with</strong> the<br />
solution defined in the experimental part. Figure 5<br />
shows the cyclic voltammetry curve resulting from<br />
this experiment.<br />
The user can find the data corresponding to this<br />
experiment in C:\ <strong>EC</strong>-<strong>Lab</strong>\ Data\ Samples\<br />
Cv_Fe_basique_1.mpr.<br />
Fig. 5: Fe(III)/Fe(II) Cyclic voltammetry.<br />
The user can press and hold the CTRL button on<br />
the keyboard to show the data point’s coordinates<br />
on the graphic window.<br />
He can process the raw data file after the<br />
experiment in order to extract specific values. A<br />
process is a function of <strong>EC</strong>-<strong>Lab</strong> Ä software (in the<br />
computer) that allows the user to calculate and<br />
display values on the graphic that are not<br />
measured by the instrument. All the processes are<br />
available in the Analysis menu. Typically in a CV<br />
protocol, the measured values are t, control, Ewe<br />
and .<br />
For example, variables that can be processed are<br />
the cycle number or the total charge variation.<br />
Processes depend on the protocol used.<br />
For cyclic voltammetry experiments, it is often<br />
necessary to do several cycles to have a real<br />
picture of the system. In fact the first cycle<br />
depends a lot on the experiment starting potential.<br />
Generally a CV experiment is made of several<br />
cycles. While processing the cycle number the<br />
user can display the cycles he wants. The way to<br />
do that is as follow:<br />
1) Process cycle number. In Analysis\ process<br />
(cycle, data…), load the CV file and click on<br />
Process. The processed file will be located in<br />
the same directory as the raw file. Its name is<br />
5
C:\<strong>EC</strong><strong>Lab</strong> \Data \samples\<br />
CV_Fe_basique_1_n.mpp.<br />
Fig. 7: CV cycle selection.<br />
Fig. 6: CV off-line process window.<br />
2) In the graphic window, right click on the mouse<br />
and choose “Selector” and “Load”. Then select<br />
the processed file in the directory<br />
3) At the right bottom corner of the graphic<br />
window one can see a frame allowing the user<br />
to highlight all or several cycles. Choose the<br />
cycle(s) you want to see (see the red circle on<br />
figure 7).<br />
After this quick start about cyclic voltammetry,<br />
another document describing voltammetric peak<br />
analysis will be soon available.<br />
Conclusion<br />
Cyclic voltammetry is the most used<br />
electrochemical technique. It is the reason why we<br />
designed this quick start <strong>with</strong> CV. Moreover<br />
<strong>EC</strong>-<strong>Lab</strong> Ä<br />
software offers lots of other abilities in<br />
general electrochemistry experiments. In addition<br />
pulsed techniques have been developed and<br />
added in <strong>EC</strong>-<strong>Lab</strong> Ä<br />
software for electroanalytical<br />
applications. A very interesting tool called protocol<br />
linker has been developed in <strong>EC</strong>-<strong>Lab</strong> Ä . It can be<br />
used to build complex experiments <strong>with</strong><br />
preconditioning steps. The user should also note<br />
that two protocols have been designed to be very<br />
modular (Modular Potentio and Modular Galvano).<br />
For more information about them, see the user<br />
manual.<br />
6