Thermal cracking of ethane in tubular reactor
Thermal cracking of ethane in tubular reactor
Thermal cracking of ethane in tubular reactor
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Thermal</strong> <strong>crack<strong>in</strong>g</strong> <strong>of</strong> <strong>ethane</strong> <strong>in</strong> <strong>tubular</strong> <strong>reactor</strong><br />
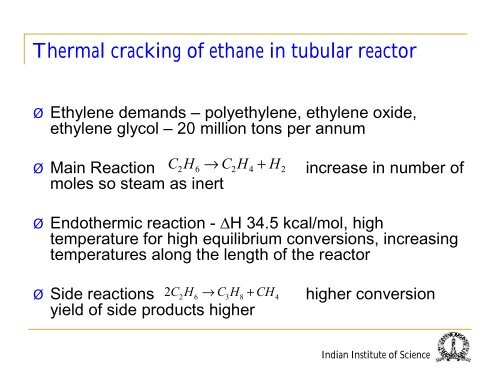
‣ Ethylene demands – polyethylene, ethylene oxide,<br />
ethylene glycol – 20 million tons per annum<br />
‣ Ma<strong>in</strong> Reaction CH → CH + H<br />
moles so steam as <strong>in</strong>ert<br />
2 6 2 4 2<br />
<strong>in</strong>crease <strong>in</strong> number <strong>of</strong><br />
‣ Endothermic reaction - ∆H 34.5 kcal/mol, high<br />
temperature for high equilibrium conversions, <strong>in</strong>creas<strong>in</strong>g<br />
temperatures along the length <strong>of</strong> the <strong>reactor</strong><br />
2C2H6 → CH<br />
3 8<br />
+ CH4<br />
‣ Side reactions<br />
yield <strong>of</strong> side products higher<br />
higher conversion<br />
Indian Institute <strong>of</strong> Science
Yield conversion diagram for <strong>ethane</strong> <strong>crack<strong>in</strong>g</strong><br />
Indian Institute <strong>of</strong> Science
Ethane <strong>crack<strong>in</strong>g</strong> <strong>reactor</strong><br />
Typical operat<strong>in</strong>g condn<br />
•L = 95 m<br />
•G = 68.68 kg/m2/s<br />
•P <strong>in</strong>let 2.99 atm, outlet 1.2 atm<br />
•T <strong>in</strong>let 680, outlet 820 C<br />
•Production 10000 tons/coil<br />
Indian Institute <strong>of</strong> Science
Balances<br />
mass<br />
dF<br />
dz<br />
j<br />
=<br />
R<br />
j<br />
4<br />
2<br />
t<br />
2<br />
dT 1 ⎡ πd<br />
t<br />
⎤<br />
energy = ⎢qz ( ) πdt + ∑−( ∆Hi)<br />
ri⎥<br />
dz ∑FC<br />
j pj ⎣<br />
4 i ⎦<br />
j<br />
πd<br />
dp ⎡2f ξ ⎤ du<br />
ρ ρ<br />
dz ⎣ d r ⎦ dz<br />
2<br />
momentum − = ⎢ + ⎥ fu +<br />
fu<br />
t<br />
π<br />
b i<br />
∑<br />
⎡RT<br />
F' 1 j<br />
u = =<br />
⎢<br />
A A⎢<br />
p<br />
⎢⎣<br />
F<br />
j<br />
⎤<br />
⎥<br />
⎥<br />
⎥⎦<br />
Indian Institute <strong>of</strong> Science
Simulation <strong>of</strong> <strong>ethane</strong> <strong>reactor</strong><br />
Indian Institute <strong>of</strong> Science
Hydrogenation <strong>of</strong> oil<br />
‣ Major demand – margar<strong>in</strong>e, shorten<strong>in</strong>gs, vanaspati<br />
‣ Vegetable oils – mixture <strong>of</strong> triglycerides - glycerol and<br />
fatty acids<br />
‣ Fatty acids – saturated (S) , monosaturated (cis, R1 and<br />
trans, R2) and diunsaturated (B). Hydrogenation to<br />
reduce odor or color, improve stability and <strong>in</strong>crease<br />
melt<strong>in</strong>g po<strong>in</strong>t.<br />
‣ Product requirements – some polyunsaturated (health)<br />
and R2 ( consistency and higher melt<strong>in</strong>g po<strong>in</strong>ts)<br />
Indian Institute <strong>of</strong> Science
Reactions<br />
1/2<br />
,<br />
1 4 H 5 6<br />
r −r ∝C r −r ∝C<br />
H<br />
2 2<br />
implies selectivity <strong>of</strong> monounsaturates over saturates<br />
proportional to (C H2 ) 1/2<br />
Indian Institute <strong>of</strong> Science
Yield conversion diagrams<br />
Indian Institute <strong>of</strong> Science
Balances<br />
dC<br />
j<br />
mass = Rj<br />
j = BR ,<br />
1, R2,<br />
M<br />
dt<br />
0<br />
2<br />
, b<br />
( ) ( )<br />
, ,<br />
,<br />
,<br />
−ka C − C = R C C<br />
dC<br />
L v H g H s H j H s<br />
H<br />
2 2 2 2<br />
( ) ( )<br />
, , , ,<br />
( )<br />
, ,<br />
1 1 1<br />
= +<br />
ka ka ka<br />
L<br />
dt<br />
v<br />
= ka C −C −ka C −C<br />
L v H g H b S S H b H s<br />
= ka C − C + R<br />
S S H b H s H<br />
2 2 2 2<br />
2 2 2<br />
L v S S<br />
Indian Institute <strong>of</strong> Science
Stirred tank batch <strong>reactor</strong><br />
‣ Desired conversion –<br />
batch time<br />
‣ Desired production rate<br />
and batch time – volume<br />
‣ Based on volume –<br />
<strong>in</strong>ternal design<br />
‣ Cool<strong>in</strong>g load<br />
∑<br />
i<br />
( ) ( )<br />
− Q = V −∆ H r = AUT −T<br />
i i K r<br />
Indian Institute <strong>of</strong> Science
Ammonia synthesis<br />
‣ Major demand – Fertilizer, chemicals,<br />
explosives, polyamides, pharmaceuticals; 150<br />
million tons per annum<br />
‣ Ma<strong>in</strong> reaction<br />
1 3<br />
N2 + H2 NH3,<br />
∆ H298K<br />
=−45.7 kJ/<br />
mol −<br />
2 2<br />
‣ High pressure, low temperatures favorable<br />
‣ Catalytic reaction – iron, promoted ruthenium<br />
1<br />
Indian Institute <strong>of</strong> Science
Ammonia synthesis – equilibrium<br />
Ammonia Mol fraction<br />
1.0<br />
0.9<br />
0.8<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
1<br />
P = 300 atm<br />
200<br />
100<br />
50<br />
10<br />
3<br />
(A)<br />
0.0<br />
500 600 700 800 900<br />
Temperature (K)<br />
Ammonia Mol fraction<br />
1.0<br />
0.9<br />
0.8<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
0.0<br />
(B)<br />
T = 473 K<br />
523<br />
573<br />
623<br />
673<br />
723<br />
773<br />
0 100 200 300<br />
Pressure (Atm)<br />
Indian Institute <strong>of</strong> Science
Ammonia synthesis - balances<br />
mass<br />
dC<br />
j<br />
u ( , )<br />
s<br />
= ηRj Cj<br />
T<br />
dz<br />
energy<br />
dT 4U<br />
ρ<br />
fuc s p<br />
= ( Tr<br />
− T) + η( −∆H)<br />
r<br />
dz d<br />
catalyst<br />
1<br />
2<br />
r dr<br />
'<br />
2 i<br />
' '<br />
ie i i<br />
'<br />
1 d 2 dT<br />
2 ⎜ r λe<br />
⎟ Hr<br />
r dr dr<br />
t<br />
d ⎛ dC ⎞<br />
⎜ rD ⎟ =−R<br />
C T<br />
⎝ dr ⎠<br />
⎛ ⎞ =∆<br />
⎝ ⎠<br />
( , )<br />
Indian Institute <strong>of</strong> Science
Reactor simulation<br />
mol fraction<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
N 2<br />
H 2<br />
NH 3<br />
0.0<br />
0 1 2 3 4 5<br />
Length (m)<br />
temperature<br />
860<br />
840<br />
820<br />
800<br />
780<br />
760<br />
740<br />
720<br />
700<br />
680<br />
0 1 2 3 4 5<br />
Length (m)<br />
120<br />
100<br />
80<br />
rate at bulk conditions<br />
observed rate<br />
rate<br />
60<br />
40<br />
20<br />
0<br />
0 1 2 3 4 5<br />
Length (m)<br />
Indian Institute <strong>of</strong> Science
Optimal temperature<br />
4//<br />
/-8<br />
34/<br />
/-7<br />
3//<br />
/-6<br />
24/<br />
Dwsdms<br />
/-5<br />
/-4<br />
/-3<br />
2//<br />
14/<br />
1//<br />
/-2<br />
04/<br />
/-1<br />
0//<br />
/-0<br />
4/<br />
34/ 4// 44/ 5// 54/ 6//<br />
Sdl odq`stqd J<br />
/<br />
Indian Institute <strong>of</strong> Science
Fixed bed <strong>reactor</strong>s<br />
Indian Institute <strong>of</strong> Science
Ammonia – Multibed <strong>reactor</strong><br />
Conversion<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
r=0<br />
r/T=0<br />
Reactor 1<br />
Reactor 2<br />
Reactor 3<br />
0.0<br />
400 500 600 700 800 900<br />
Temperature<br />
Indian Institute <strong>of</strong> Science
Fixed bed <strong>reactor</strong>s<br />
Indian Institute <strong>of</strong> Science
Ammonia – Autothermal<br />
300<br />
1000<br />
T top<br />
-T 1L<br />
DT<br />
250<br />
200<br />
150<br />
100<br />
T top<br />
-T feed<br />
473<br />
491<br />
513<br />
523<br />
578<br />
623<br />
T feed<br />
K<br />
900<br />
800<br />
700<br />
600<br />
50<br />
500<br />
0<br />
400 500 600 700 800 900 1000<br />
T top<br />
400<br />
400 500 600 700 800 900 1000<br />
T top<br />
K<br />
Indian Institute <strong>of</strong> Science
Ammonia – Autothermal<br />
800<br />
750<br />
Conversion<br />
700<br />
650<br />
600<br />
550<br />
<strong>reactor</strong><br />
heat exchanger<br />
Conversion<br />
0.8<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
r=0<br />
∂r/∂T=0<br />
500<br />
0 1 2 3 4 5 6<br />
Length<br />
0.1<br />
0.0<br />
600 650 700 750 800 850 900<br />
Temperature<br />
Indian Institute <strong>of</strong> Science