Thin Flexible Linear Polarizer of Silver-Dispersed Polyimide Blend ...
Thin Flexible Linear Polarizer of Silver-Dispersed Polyimide Blend ...
Thin Flexible Linear Polarizer of Silver-Dispersed Polyimide Blend ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Thin</strong> <strong>Flexible</strong> <strong>Linear</strong> <strong>Polarizer</strong> <strong>of</strong> <strong>Silver</strong>-<strong>Dispersed</strong><br />
<strong>Polyimide</strong> <strong>Blend</strong> Film Exhibiting<br />
High Extinction Ratios in Near-IR Region<br />
S. Matsuda and S. Ando<br />
Department <strong>of</strong> Organic and Polymeric Materials, Tokyo Institute <strong>of</strong> Technology, Tokyo, Japan<br />
smatsuda@polymer.titech.ac.jp<br />
Abstract: <strong>Flexible</strong> thin-film (19 µm-thich) polarizers consisting <strong>of</strong> silver nanoparticles having<br />
rodlike shapes dispersed in polyimide blend films were prepared. <strong>Linear</strong> polarizing character with<br />
extinction ratios >20dB and transparencies >75% were obtained in a wide-range <strong>of</strong> wavelengths<br />
(1.4-1.8 µm).<br />
©2005 Optical Society <strong>of</strong> America<br />
OCIS codes: (160.1190) Anisotropic optical materials; (160.5470) Polymers<br />
1. Introduction<br />
Conventional thin-film polarizers for optical communication devices are made <strong>of</strong> stretched inorganic glass containing<br />
silver (Ag) particles [1,2], periodic metal-silica layers [3], and elongated Ag islands embedded in glasses [4]. Although<br />
such inorganic materials exhibit high optical extinction ratios and good thermal stability, they are not easy to make<br />
into thin plates or small components due to their brittleness. Conventional polymeric polarizers containing iodine or<br />
dichroic dyes have good flexibility and tractability, however, the polymeric polarizers exhibit no polarizing character<br />
in the near-infrared (IR) region (>0.7 µm) and no thermal stability above 100°C. In addition, the thicknesses <strong>of</strong><br />
polymeric polarizers are larger than 50 µm, which should result in considerable excess losses when they are inserted<br />
into waveguide gaps. We have reported that distinct optical anisotropies in the visible and near-IR region (0.6−0.9<br />
µm) are generated in a precursor films <strong>of</strong> rigid-rod fluorinated polyimide (PI) dissolving silver nitrate (AgNO 3<br />
) during<br />
thermal curing and uniaxial drawing [5]. In addition, we have developed thin-film PI polarizers containing silver<br />
nanoparticles having spheroidal shape [6,7]. The PI polarizers have good tractability and high thermal stability (>200°C),<br />
but the polarizing efficiency is not sufficiently high above 1.0 µm. Hence, there has been a demand for a 10–20 µmthick<br />
polarizer applicable to optical communication wavelength region (1.3−1.7 µm) while having good processability<br />
and tractability. In this study, we investigate the precipitation behavior <strong>of</strong> silver nanoparticles and the generation <strong>of</strong><br />
optical anisotropy in uniaxially drawn PI blend films. In addition, by optimizing the preparing conditions, we<br />
successfully fabricated novel thin-film PI blend polarizers having high extinction ratios (22dB) and transparency<br />
(77%) at 1.55 µm.<br />
2. Experimental<br />
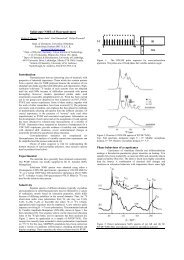
Fig. 1 schematically shows a method for fabricating a PI blend polarizer. A rigid-rod fluorinated PI, PMDA/TFDB<br />
(PI-1) synthesized from pyromellitic dianhydride (PMDA) and 2,2'-bis-(trifluoromethyl)-4,4'-diaminobiphenyl (TFDB)<br />
was used as a continuous phase, and a rigid-rod sulfonated PI, PMDA/BDSA (PI-2) synthesized from PMDA and<br />
2,2’-benzidinedisulfonic acid (BDSA) was used as a dispersed phases in the PI blend system. These two PIs show<br />
macro-phase separation. AgNO 3<br />
(Aldrich) was used as a metallic dopant. A poly(amic acid) (PAA) <strong>of</strong> PI-2, a precursor<br />
Ag nanoparticles<br />
PI-1<br />
+<br />
PI-2 + Ag<br />
//<br />
Heating<br />
&<br />
Drawing<br />
Heating<br />
⊥<br />
(a)<br />
(b)<br />
(c)<br />
Fig. 1. A fabrication scheme <strong>of</strong> a thin-film PI blend polarizer. The fluorinated PI-1 and the sulfonated PI-2 exhibit macrophase<br />
separation in the film, and nanocrystalline Ag-particles are selectively precipitated in the PI-2 phase due to the difference<br />
in polarity and the specific interactions between Ag + ions and sulfonic acid. The PI-2 phase works as nanoreacters for Ag +<br />
reduction and anisotropic growth <strong>of</strong> Ag particles.
T //<br />
100<br />
80<br />
(a) 30 min<br />
(b) 60 min<br />
60<br />
T T<br />
40 ⊥<br />
⊥<br />
T<br />
20 //<br />
20<br />
T //<br />
T //<br />
0<br />
100<br />
80<br />
(c) 90 min<br />
(d) 120 min<br />
60<br />
40 T⊥<br />
T ⊥<br />
0<br />
0.4 0.8 1.2 1.6 0.4 0.8 1.2 1.6<br />
Wavelength (µm)<br />
Fig. 2. Polarized transmission spectra <strong>of</strong> Ag-dispersed PI blend films with varying t H<br />
= (a)30, (b)60, (c)90, and (d)120 min at<br />
T f<br />
=345°C for normal incident light polarized parallel (T //<br />
) and perpendicular (T ⊥<br />
) to the drawing direction.<br />
<strong>of</strong> PI, was added to a PAA <strong>of</strong> PI-1 (in DMAc solution, 10 wt%, 300 Poise) and stirred for 12 h at room temperature,<br />
followed by addition <strong>of</strong> AgNO 3<br />
to the solution and stirring for 12 h. Most <strong>of</strong> Ag + ions are expected to be transferred<br />
and included in the PI-2 phase due to the difference <strong>of</strong> polarity between the two PIs and the specific interactions<br />
between Ag + and sulfonic acid [8]. The stirred solution were spin-coated onto a 4-inch silicon wafer, followed by<br />
drying in N 2<br />
at 70°C for 1 h. PAA films peeled from substrate were uniaxially drawn up to 1.7 times <strong>of</strong> draw ratio<br />
during thermal curing with a constant tensile load using a thermomechanical analyzer (TMA, Sinku-Riko TM-7000)<br />
or an automatic thermal drawing machine equipped in our laboratory. The PAA films were heated to the final curing<br />
temperature (T f<br />
) at 10°C/min, kept at the temperature for a constant holding time (t H<br />
), and then cooled to room<br />
temperature. The thermal curing was carried out in air. Ag nanoparticles are precipitated in PI blend films during<br />
thermal curing. Polarized optical transmittance spectra <strong>of</strong> the PI blend films thus obtained were measured by rotating<br />
a Glan-Taylor prism (polarizer) inserted into the light path <strong>of</strong> a spectrophotometer (Hitachi U-3500, λ= 0.3-2.5 µm).<br />
Cross-sectional transmission electron micrographs (TEMs) were taken with JEOL 100CX TEM. The uniaxially<br />
drawn PI blend films were embedded in epoxy resin and then sectioned into 60 nm thicknesses with a Reichert-Jung<br />
Werke ultramicrotome. The sectioning directions were parallel to the drawing direction.<br />
3. Results and Discussion<br />
Fig. 2 shows the polarized transmission spectra <strong>of</strong> PI blend films with varying the holding time (t H<br />
=30, 60, 90, and 120<br />
min) at a final curing temperature (T f<br />
= 345°C). The molar ratios <strong>of</strong> PI-1, PI-2, and AgNO 3<br />
in the blend films were<br />
100/0.95/6. During thermal curing <strong>of</strong> a uniaxially drawn PI blend film, the spectral shapes along the two polarizations<br />
(parallel and perpendicular to the drawing direction) were gradually changed due to the precipitation <strong>of</strong> Ag nanoparticles.<br />
Strong extinctions with a small anisotropy, which is caused by the plasmon resonance <strong>of</strong> spherical Ag nanoparticles,<br />
are observed around λ=0.5 µm for the film cured for t H<br />
=30 min. As t H<br />
increases, an extinction peak in T //<br />
was gradually<br />
shifted to a longer wavelength and the peak-top finally reaches to λ=1.0 µm at t H<br />
=120 min. On the other hand, T ⊥<br />
gradually decreased in a wide wavelength region (0.4 < λ < 1.6 µm) until t H<br />
= 90 min and then increased at t H<br />
= 120<br />
min. As a result, high polarizing efficiency (i.e. high extinction ratios) was obtained in the region <strong>of</strong> ca. 0.9-1.2 µm for<br />
the film cured for t H<br />
=120 min. The wavelength region with high extinction ratios is located at much longer than that<br />
had been obtained for a uniaxially drawn neat PI-1 film containing Ag nanoparticles [7]. In addition, a similar<br />
Fig. 3. Cross-sectional TEM images <strong>of</strong> the Ag-dispersed PI blend film cured for 120 min at T f<br />
= 345°C. Arrows denote the<br />
drawing direction. The inset shows the magnified image <strong>of</strong> a typical Ag nanoparticle.
(a)<br />
100<br />
80<br />
60<br />
40<br />
T ⊥<br />
22 dB<br />
@1.55 m<br />
(b)<br />
30<br />
20<br />
T //<br />
0<br />
0.5 1.0 1.5 2.0 2.5<br />
0 0.5 1.0 1.5 2.0 2.5<br />
Wavelength (µm)<br />
Wavelength (µm)<br />
Fig. 4. (a) Polarized transmission spectra <strong>of</strong> uniaxially drawn Ag-dispersed PI blend film exhibiting a large extinction ratio at<br />
1.55 µm and (b) the spectrum <strong>of</strong> extinction ratio calculated from the polarized transmission spectra. The inset shows the<br />
aspect <strong>of</strong> the film.<br />
phenomenon was observed in case <strong>of</strong> the films cured with varying T f<br />
values. The desirable t H<br />
value for obtaining high<br />
polarizing efficiency decreases as the T f<br />
value increases, whereas the extinction ratio increases.<br />
The cross-sectional TEM images <strong>of</strong> a PI blend film cured for t H<br />
=120 min at T f<br />
=345°C are shown in Fig. 3. Ag<br />
nanoparticles having rodlike shapes whose longer axes are directed to the drawing direction are observed (see inset<br />
magnified image). The diameters <strong>of</strong> the Ag nanoparticles along their longer axes are 100∼200 nm, and their aspect<br />
ratios are ca. 4. In our previous study, the corresponding values were ca. 30 nm and 1.5, respectively for uniaxially<br />
drawn neat PI-1 films [7]. This indicates that considerably larger and more elongated Ag nanoparticles are precipitated<br />
in the PI blend film. These results strongly suggests that Ag + ions are selectively included in the PI-2 phase during<br />
stirring <strong>of</strong> PI blend solution, and Ag nanoparticles are mainly precipitated in deformed (elliptic-shaped) PI-2 islands<br />
containing concentrated Ag + ions.<br />
Fig. 4 shows the polarized transmission spectra <strong>of</strong> a PI blend film fabricated with the optimized preparing conditions,<br />
i.e. the mol ratio <strong>of</strong> PI-1/PI-2/AgNO 3<br />
(100/1.5/9.75), T f<br />
(420°C) and t H<br />
(5 min). High polarizing efficiency with<br />
extinction ratios larger than 20dB (22dB at λ = 1.55 µm) and transparencies higher than 75% (T ⊥<br />
= 77% at λ = 1.55<br />
µm; including surface reflection) was obtained in a wide wavelength region (1.4 < λ < 1.8 µm). The thickness <strong>of</strong> the<br />
film is sufficiently thin as 19 µm and shows good flexibility and tractability (see the inset in Fig. 4).<br />
4. Summary<br />
The precipitation behavior <strong>of</strong> Ag nanoparticles in uniaxially drawn PI blend films and their optical anisotropy were<br />
investigated, and we have successfully fabricated a novel flexible thin-film polarizer for optical communication<br />
wavelengths. The Ag nanoparticles precipitated in a PI blend film are much larger and more elongated than those<br />
precipitated in a neat PI film, which results in the significant optical anisotropy with high extinction ratios. The PI<br />
blend film fabricated under the optimized condition exhibits high polarizing efficiency in a wide wavelength region<br />
(1.4 < λ < 1.8 µm). In addition, the thickness <strong>of</strong> 19 µm is sufficiently thin for reducing any excess losses caused by<br />
insertion <strong>of</strong> film polarizers into optical devices. The film is tough and flexible, easy for insertion. Hence, the material<br />
system based on novel PI blends and the fabrication procedures designed in this study are promising for fabricating<br />
flexible thin-film polarizers for optical waveguide devices.<br />
References<br />
[1] S.D. Stookey and R.J. Araujo, “Selective polarization <strong>of</strong> light due to absorption by small elongated silver particles<br />
in glass”, Appl. Opt. 7, 777-779 (1968).<br />
[2] D.N. Gritz, “Near-infrared (IR) polarizing glass” in Infrared Systems and Components, R.L. Caswell ed., Proc.<br />
SPIE 750, 18-26 (1987).<br />
[3] S. Kawakami, “Light propagation along periodic metal-dielectric layers”, Appl. Opt. 22, 2426-2428 (1983).<br />
[4] K. Baba and M. Miyagi, “Optical polarizer using anisotropic metallic island films with a large aperture and a high<br />
extinction ratio”, Opt. Lett. 16, 964-966 (1991).<br />
[5] T. Sawada, S. Ando, and S. Sasaki, “Optical anisotropy <strong>of</strong> uniaxially drawn and silver-dispersed polyimide films”,<br />
Appl. Phys. Lett. 74, 938-940 (1999).<br />
[6] S. Matsuda, S. Ando, and T. Sawada, “<strong>Thin</strong> flexible polariser <strong>of</strong> Ag-nanoparticle-dispersed fluorinated polyimide”,<br />
Electron. Lett. 37, 706-707 (2001).<br />
[7] S. Matsuda and S. Ando, “Anisotropy in optical transmittance and molecular chain orientation <strong>of</strong> silver-dispersed<br />
uniaxially drawn polyimide films”, Polym. Adv. Technol. 14, 458-470 (2003).<br />
[8] S. Horiuchi, T. Fujita, T. Hayakawa, and Y. Nakao, “Three-dimensional nanoscale alignment <strong>of</strong> metal nanoparticles<br />
using block copolymer films as nanoreactors”, Langmuir 19, 2963-2973 (2003).<br />
20<br />
10