Synthesis of Sulfonated Poly(1,4-diphenoxybenzene) for Proton ...
Synthesis of Sulfonated Poly(1,4-diphenoxybenzene) for Proton ...
Synthesis of Sulfonated Poly(1,4-diphenoxybenzene) for Proton ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
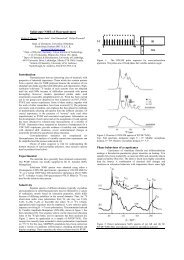
<strong>Sulfonated</strong> <strong>Poly</strong>(1,4-<strong>diphenoxybenzene</strong>) <strong>for</strong> <strong>Proton</strong> Exchange Membrane<strong>Sulfonated</strong> <strong>Poly</strong> (1,4-diphenoxyphenylene) (4)To a solution <strong>of</strong> polymer 2 (0.13 g, 0.5 mmol) in dichloromethane(2 mL), a solution <strong>of</strong> chlorosulfonicacid (0.13 mL, 2 mmol) in dichloromethane (0.8 mL)was slowly added with stirring under nitrogen. Themixture was stirred at room temperature <strong>for</strong> 24 h,and then poured into hexane. The precipitate was collectedby filtration and stirred in hot toluene. The precipitatewas collected and washed with toluene andhexane. It was dried at 100 C <strong>for</strong> 8 h under the reducedpressure to give the fully sulfonated polymer4a. The yield was 0.19 g (90%). IR (KBr, ); 1184(-SO 3 H), 1223 (C-O-C), 690, 1493, 1601 cm 1 (Ph).The partially sulfonated polymers 4b and 4c were preparedby using 1.00 (0.07 mL) and 0.5 (0.03 mL)mmol <strong>of</strong> chlorosulfonic acid, respectively in the similarconditions described above. The numbers <strong>of</strong> sulfonicacid groups in the repeating unit <strong>of</strong> 4b and4c were 0.74 and 0.38, respectively. Ion exchangecapacity (IEC) was determined by titration with0.02 M NaOH aq.MeasurementsThe infrared spectra were recorded on a Horiba FT-720 spectrophotometer. The 1 H and 13 C NMR spectrawere obtained on a JEOL JNM-AL400 spectrometerat 1 H, 400 MHz and 13 C, 100 MHz. Number- andweight-average molecular weights (M n and M w ) weredetermined by a gel permeation chromatograph (GPC)on a Hitachi LC-7000 system equipped with a polystyrenegel column (TSKgel GMHHR-M) eluted withtetrahydr<strong>of</strong>uran (THF) at a flow rate <strong>of</strong> 1.0 mL min 1calibrated by standard polystyrene samples. The cyclicvoltammogram was measured at room temperaturein a typical three electrode with a working (Ptwire), a reference (Ag/AgCl), and a counter electrode(Pt spiral) under a nitrogen atmosphere at a sweepingrate <strong>of</strong> 10 mV/s (Hokuto Denko HSV-100). A 0.1 Msolution <strong>of</strong> tetrabutylammonium perchlorate in anhydrousacetonitrile was used as an electrolyte. Thermalanalysis was per<strong>for</strong>med on a Seiko EXSTAR 6000TG/DTA 6300 thermal analyzer at a heating rate <strong>of</strong>10 C/min <strong>for</strong> thermogravimetry (TG), differentialthermal analysis (DTA), and a Seiko EXSTAR 6000DSC 6200 at a heating rate <strong>of</strong> 10 C/min in the temperaturerange from 25 to 300 C <strong>for</strong> differential scanningcalorimetry (DSC) under nitrogen. Disk-likesamples with thickness <strong>of</strong> 600 mm were prepared bycompression molding. <strong>Proton</strong> conductivity measurementswere per<strong>for</strong>med on the acid <strong>for</strong>m <strong>of</strong> the disklikesamples using a Hioki 3532-80 Chemical impedancemeter. An impedance spectrum was recordedfrom 5 Hz to 1 MHz. The resistance <strong>of</strong> the samplewas taken at the frequency that produced the minimumimaginary response. All the impedance measurementswere per<strong>for</strong>med at 80 C above water with 95%or 50% relative humidity (RH).CalculationThe density functional theory (DFT) with the threeparameterBecke-style hybrid functionals (B3LYP)was used <strong>for</strong> calculation <strong>of</strong> the Mulliken populationcharges <strong>for</strong> neutral compounds and the local spin densities<strong>for</strong> radical cations. Molecular geometries in theneutral sates were optimized under no constraints, andthe Fukui functions were calculated as differences inMulliken charges between the neutral and the radicalcation states having the same molecular geometries.The 6-311G(d) basis set was used <strong>for</strong> the geometryoptimization and the calculations <strong>of</strong> Mulliken chargesand spin densities. Radical cations were always treatedas open shell systems (uB3LYP). The moleculargeometries <strong>of</strong> radical cations were optimized independentlyfrom the neutral molecules prior to the calculations<strong>of</strong> local spin densities. In addition, the UV/Vis optical absorption spectra were calculated <strong>for</strong>monomer (1) and model compounds using the timedependentDFT methods with the B3LYP functionaland the 6-311 þþ G(d,p) basis set. The details <strong>for</strong> thetheoretical prediction <strong>of</strong> absorption spectra have beenreported elsewhere. 8 All the calculations were per<strong>for</strong>medusing the s<strong>of</strong>tware <strong>of</strong> Gaussian-03 (RevisionC02). 11RESULTS AND DISCUSSIONCyclic Voltammogram <strong>of</strong> Monomer 1It is important to measure the oxidation-reductionpotentials <strong>of</strong> 1 <strong>for</strong> the selection <strong>of</strong> oxidants. The cyclicvoltammogram <strong>of</strong> 1 was measured in acetonitrile solutioncontaining 0.1 M tetrabutylammonium perchloratein a cell equipped with a reference electrode(Ag/AgCl), a working electrode (Pt), and an auxiliaryelectrode (Pt). The redox process was observed at theoxidation potential value <strong>of</strong> 1.6 V vs SCE (Figure 1).Thus, FeCl 3 was selected as the oxidant <strong>for</strong> oxidativecoupling polymerization <strong>of</strong> 1.-2 -1 0 1 1.63 2V (vs Ag/AgCl)Figure 1. Cyclic voltammogram <strong>of</strong> monomer 1.<strong>Poly</strong>m. J., Vol. 39, No. 8, 2007 883
<strong>Sulfonated</strong> <strong>Poly</strong>(1,4-<strong>diphenoxybenzene</strong>) <strong>for</strong> <strong>Proton</strong> Exchange MembraneSO 3 HOOOOFeCl 3ClSO 3 HNitrobenzene, 24hn CH 2 Cl 2nOO1 2 4aHO 3 SScheme 1.Oxidative coupling polymerization <strong>of</strong> monomer 1 and sulfonation <strong>of</strong> the resulting polymer.Figure 2.DMSO-d 6 .Expanded 13 C NMR spectra <strong>of</strong> 1 and polymer 2 inFigure 3.DMSO-d 6 .Expanded 13 C NMR spectra <strong>of</strong> 3 and polymer 4a in2 are shown in Figure 2. The 13 C NMR signal <strong>of</strong> polymer2 is very similar to that <strong>of</strong> monomer 1, but it isdifficult to determine the coupling positions because13 C peaks <strong>of</strong> coupling positions are too weak to identify.Thus, to estimate the structure <strong>of</strong> polymer 2,a model compound, p-phenylenedioxybis(4-benzenesulfonicacid) (3) was prepared by sulfonation <strong>of</strong> 1because the para position (1) <strong>of</strong> the phenoxy groupin 1 would be the other probable coupling position.The structure <strong>of</strong> 3 was confirmed by IR and NMRspectroscopy and elemental analysis. However, thecoupling reaction <strong>of</strong> position (1) <strong>of</strong> the phenoxy groupin 1 cannot be completely excluded because <strong>of</strong> week13 C peaks <strong>of</strong> coupling positions.<strong>Synthesis</strong> <strong>of</strong> <strong>Poly</strong>mer 4aSulfonation <strong>of</strong> polymer 2 was also carried out toshow that the para position (1) <strong>of</strong> the phenoxy groupwas not involved in the coupling (Scheme 1).Figure 3 shows the 13 C NMR spectra <strong>of</strong> 3 and polymer4a. The C-1 resonance <strong>of</strong> 122.96 ppm in 1 shiftsto 141.57 ppm in 3, and the C-1’ resonance <strong>of</strong> 123.11ppm in polymer 2 also shifts to 141.31 ppm in polymer4a. All other peaks are well assigned to the proposedstructure <strong>of</strong> 3 and polymer 4a. These findingsindicate the C-1 position in 1 is almost intact duringthe polymerization and is selectively sulfonated bythe subsequent treatment with chlorosulfonic acid.These reacted positions are in very good agreementwith those predicted by the DFT calculations.The UV/Vis absorption spectra <strong>of</strong> monomer 1 andpolymer 2 in chlor<strong>of</strong>orm with the concentrations <strong>of</strong>5 10 5 mol/L was measured. The absorption edge( e )<strong>of</strong>2 appears at 550 nm, which is much longerwavelengths than 1, suggesting the delocalization <strong>of</strong>-electrons (-conjugation) along the polymer mainchain (Figure 4). <strong>Poly</strong>mer 2 was soluble in tetrahydr<strong>of</strong>uran,chlor<strong>of</strong>orm and nitrobenzene at room temperature.On the other hand, polymer 4a was soluble inwater and polar aprotic solvents, such as N,N-dimethyl<strong>for</strong>mamide,N,N-dimethylacetamide, and dimethylsulfoxide at room temperature. The thermal properties<strong>of</strong> polymer 2 were examined by thermogravimetry(TG) and differential scanning calorimetry (DSC).<strong>Poly</strong>m. J., Vol. 39, No. 8, 2007 885
<strong>Sulfonated</strong> <strong>Poly</strong>(1,4-<strong>diphenoxybenzene</strong>) <strong>for</strong> <strong>Proton</strong> Exchange MembraneAdamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O.Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W.Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P.Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich,A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D.Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q.Cui, A. G. Baboul, S. Clif<strong>for</strong>d, J. Cioslowski, B. B.Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi,R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y.Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B.Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A.Pople, Gaussian, Inc., Walling<strong>for</strong>d CT, 2004.<strong>Poly</strong>m. J., Vol. 39, No. 8, 2007 887