Atom transfer radical polymerizations of styrene and butadiene as ...
Atom transfer radical polymerizations of styrene and butadiene as ...
Atom transfer radical polymerizations of styrene and butadiene as ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
46 J. Hua et al.<br />
8 6 4 2 0 ppm<br />
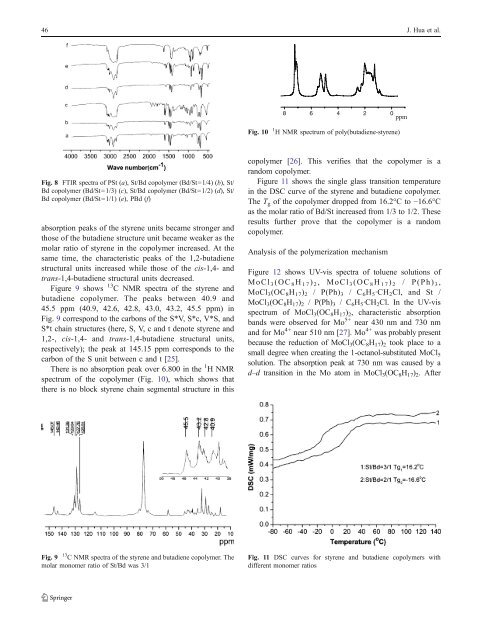
Fig. 10 1 H NMR spectrum <strong>of</strong> poly(<strong>butadiene</strong>-<strong>styrene</strong>)<br />
Fig. 8 FTIR spectra <strong>of</strong> PSt (a), St/Bd copolymer (Bd/St=1/4) (b), St/<br />
Bd copolymer (Bd/St=1/3) (c), St/Bd copolymer (Bd/St=1/2) (d), St/<br />
Bd copolymer (Bd/St=1/1) (e), PBd (f)<br />
absorption peaks <strong>of</strong> the <strong>styrene</strong> units became stronger <strong>and</strong><br />
those <strong>of</strong> the <strong>butadiene</strong> structure unit became weaker <strong>as</strong> the<br />
molar ratio <strong>of</strong> <strong>styrene</strong> in the copolymer incre<strong>as</strong>ed. At the<br />
same time, the characteristic peaks <strong>of</strong> the 1,2-<strong>butadiene</strong><br />
structural units incre<strong>as</strong>ed while those <strong>of</strong> the cis-1,4- <strong>and</strong><br />
trans-1,4-<strong>butadiene</strong> structural units decre<strong>as</strong>ed.<br />
Figure 9 shows 13 C NMR spectra <strong>of</strong> the <strong>styrene</strong> <strong>and</strong><br />
<strong>butadiene</strong> copolymer. The peaks between 40.9 <strong>and</strong><br />
45.5 ppm (40.9, 42.6, 42.8, 43.0, 43.2, 45.5 ppm) in<br />
Fig. 9 correspond to the carbons <strong>of</strong> the S*V, S*c, V*S, <strong>and</strong><br />
S*t chain structures (here, S, V, c <strong>and</strong> t denote <strong>styrene</strong> <strong>and</strong><br />
1,2-, cis-1,4- <strong>and</strong> trans-1,4-<strong>butadiene</strong> structural units,<br />
respectively); the peak at 145.15 ppm corresponds to the<br />
carbon <strong>of</strong> the S unit between c <strong>and</strong> t [25].<br />
There is no absorption peak over 6.800 in the 1 HNMR<br />
spectrum <strong>of</strong> the copolymer (Fig. 10), which shows that<br />
there is no block <strong>styrene</strong> chain segmental structure in this<br />
copolymer [26]. This verifies that the copolymer is a<br />
r<strong>and</strong>om copolymer.<br />
Figure 11 shows the single gl<strong>as</strong>s transition temperature<br />
in the DSC curve <strong>of</strong> the <strong>styrene</strong> <strong>and</strong> <strong>butadiene</strong> copolymer.<br />
The T g <strong>of</strong> the copolymer dropped from 16.2°C to −16.6°C<br />
<strong>as</strong> the molar ratio <strong>of</strong> Bd/St incre<strong>as</strong>ed from 1/3 to 1/2. These<br />
results further prove that the copolymer is a r<strong>and</strong>om<br />
copolymer.<br />
Analysis <strong>of</strong> the polymerization mechanism<br />
Figure 12 shows UV-vis spectra <strong>of</strong> toluene solutions <strong>of</strong><br />
MoCl 3 (OC 8 H 17 ) 2 , MoCl 3 (OC 8 H 17 ) 2 / P(Ph) 3 ,<br />
MoCl 3 (OC 8 H 17 ) 2 / P(Ph) 3 / C 6 H 5·CH 2 Cl, <strong>and</strong> St /<br />
MoCl 3 (OC 8 H 17 ) 2 / P(Ph) 3 / C 6 H 5·CH 2 Cl. In the UV-vis<br />
spectrum <strong>of</strong> MoCl 3 (OC 8 H 17 ) 2 , characteristic absorption<br />
b<strong>and</strong>s were observed for Mo 5+ near 430 nm <strong>and</strong> 730 nm<br />
<strong>and</strong> for Mo 4+ near 510 nm [27]. Mo 4+ w<strong>as</strong> probably present<br />
because the reduction <strong>of</strong> MoCl 3 (OC 8 H 17 ) 2 took place to a<br />
small degree when creating the 1-octanol-substituted MoCl 5<br />
solution. The absorption peak at 730 nm w<strong>as</strong> caused by a<br />
d–d transition in the Mo atom in MoCl 3 (OC 8 H 17 ) 2 .After<br />
Fig. 9 13 C NMR spectra <strong>of</strong> the <strong>styrene</strong> <strong>and</strong> <strong>butadiene</strong> copolymer. The<br />
molar monomer ratio <strong>of</strong> St/Bd w<strong>as</strong> 3/1<br />
Fig. 11 DSC curves for <strong>styrene</strong> <strong>and</strong> <strong>butadiene</strong> copolymers with<br />
different monomer ratios