Chapter 4.pdf
Chapter 4.pdf
Chapter 4.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The temperature at which a solid turns into a liquid is called<br />
the melting point. For example, the melting point of water is<br />
0°C. The reverse process, freezing, occurs at the freezing point.<br />
The melting point and the freezing point are always the same<br />
temperature. Similarly, the temperature at which a liquid turns to<br />
a gas is called the boiling point. The boiling point is the same<br />
temperature as the condensing point, the temperature at which a<br />
gas changes into a liquid.<br />
The Particle Theory of Matter<br />
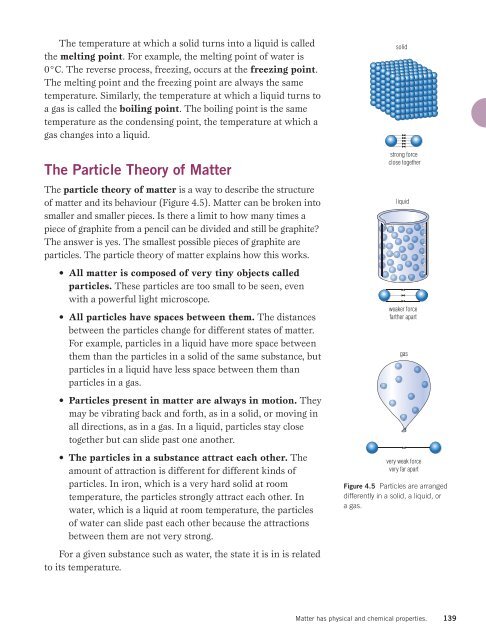
The particle theory of matter is a way to describe the structure<br />
of matter and its behaviour (Figure 4.5). Matter can be broken into<br />
smaller and smaller pieces. Is there a limit to how many times a<br />
piece of graphite from a pencil can be divided and still be graphite<br />
The answer is yes. The smallest possible pieces of graphite are<br />
particles. The particle theory of matter explains how this works.<br />
• All matter is composed of very tiny objects called<br />
particles. These particles are too small to be seen, even<br />
with a powerful light microscope.<br />
• All particles have spaces between them. The distances<br />
between the particles change for different states of matter.<br />
For example, particles in a liquid have more space between<br />
them than the particles in a solid of the same substance, but<br />
particles in a liquid have less space between them than<br />
particles in a gas.<br />
• Particles present in matter are always in motion. They<br />
may be vibrating back and forth, as in a solid, or moving in<br />
all directions, as in a gas. In a liquid, particles stay close<br />
together but can slide past one another.<br />
• The particles in a substance attract each other. The<br />
amount of attraction is different for different kinds of<br />
particles. In iron, which is a very hard solid at room<br />
temperature, the particles strongly attract each other. In<br />
water, which is a liquid at room temperature, the particles<br />
of water can slide past each other because the attractions<br />
between them are not very strong.<br />
For a given substance such as water, the state it is in is related<br />
to its temperature.<br />
solid<br />
strong force<br />
close together<br />
liquid<br />
weaker force<br />
farther apart<br />
gas<br />
very weak force<br />
very far apart<br />
Figure 4.5 Particles are arranged<br />
differently in a solid, a liquid, or<br />
a gas.<br />
Matter has physical and chemical properties.<br />
139