EpiTan Ltd. - Clinuvel Pharmaceuticals
EpiTan Ltd. - Clinuvel Pharmaceuticals
EpiTan Ltd. - Clinuvel Pharmaceuticals
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Oct. 07, 2004 7:04<br />
<strong>EpiTan</strong> <strong>Ltd</strong>.<br />
<strong>Pharmaceuticals</strong><br />
> Click here for Disclaimer<br />
Lead Drug „Melanotan“ sizzles with<br />
Blockbuster Potential<br />
Rating:<br />
Speculative Buy since: 07 Oct 2004<br />
Share price target: AUD 3.50<br />
since: 07 Oct 2004<br />
Current price: AUD 0.87<br />
High/Low 52W: AUD 1.08/0.57<br />
Figures per share in AUD<br />
2004 2005e 2006e<br />
EPS -0.07 -0.02 -0.02<br />
P/E n.m. n.m. n.m.<br />
CashFlow -0.11 -0.01 -0.01<br />
P/CF n.m. n.m. n.m.<br />
Dividend n.m. n.m. n.m.<br />
Yield n.m. n.m. n.m.<br />
Book Value 0.08 0.05 0.03<br />
Cash 0.05 0.07 0.13<br />
Fiscal Year as of 30.06.<br />
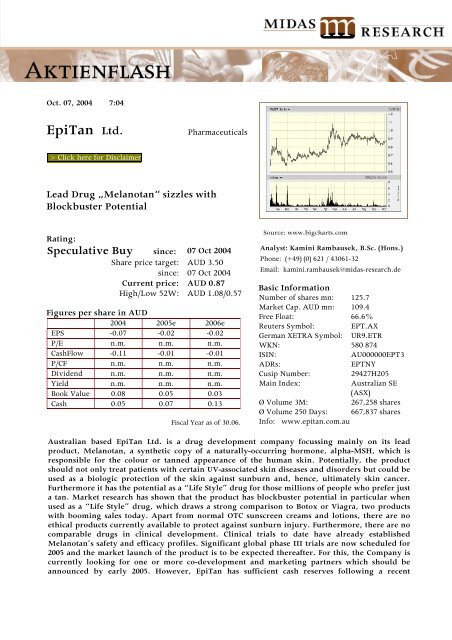
Source: www.bigcharts.com<br />
Analyst: Kamini Rambausek, B.Sc. (Hons.)<br />
Phone: (+49) (0) 621 / 43061-32<br />
Email: kamini.rambausek@midas-research.de<br />
Basic Information<br />
Number of shares mn: 125.7<br />
Market Cap. AUD mn: 109.4<br />
Free Float: 66.6%<br />
Reuters Symbol: EPT.AX<br />
German XETRA Symbol: UR9.ETR<br />
WKN: 580 874<br />
ISIN:<br />
AU000000EPT3<br />
ADRs:<br />
EPTNY<br />
Cusip Number: 29427H205<br />
Main Index:<br />
Australian SE<br />
(ASX)<br />
Ø Volume 3M:<br />
267,258 shares<br />
Ø Volume 250 Days: 667,837 shares<br />
Info: www.epitan.com.au<br />
Australian based <strong>EpiTan</strong> <strong>Ltd</strong>. is a drug development company focussing mainly on its lead<br />
product, Melanotan, a synthetic copy of a naturally-occurring hormone, alpha-MSH, which is<br />
responsible for the colour or tanned appearance of the human skin. Potentially, the product<br />
should not only treat patients with certain UV-associated skin diseases and disorders but could be<br />
used as a biologic protection of the skin against sunburn and, hence, ultimately skin cancer.<br />
Furthermore it has the potential as a “Life Style” drug for those millions of people who prefer just<br />
a tan. Market research has shown that the product has blockbuster potential in particular when<br />
used as a “Life Style” drug, which draws a strong comparison to Botox or Viagra, two products<br />
with booming sales today. Apart from normal OTC sunscreen creams and lotions, there are no<br />
ethical products currently available to protect against sunburn injury. Furthermore, there are no<br />
comparable drugs in clinical development. Clinical trials to date have already established<br />
Melanotan’s safety and efficacy profiles. Significant global phase III trials are now scheduled for<br />
2005 and the market launch of the product is to be expected thereafter. For this, the Company is<br />
currently looking for one or more co-development and marketing partners which should be<br />
announced by early 2005. However, <strong>EpiTan</strong> has sufficient cash reserves following a recent
financing round, to at least initiate further clinical studies should they have difficulty finding an<br />
appropriate pharmaceutical partner in the next 12 months.<br />
<strong>EpiTan</strong> - Corporate Profile<br />
Based in Melbourne (Australia) and listed on the Australian Stock Exchange (Melbourne) since February<br />
2001, <strong>EpiTan</strong> Limited is a pharmaceutical company with its primary focus on the development and<br />
commercialisation of its lead product, Melanotan. The product was originally developed in the 1980’s at<br />
the University of Arizona, USA. In 1995, the Melanotan Corporation Inc. was formed to seek a pharma<br />
company to further develop and commercialise Melanotan and <strong>EpiTan</strong> began negotiating with this Group<br />
to obtain the exclusive rights to the drug. <strong>EpiTan</strong> successfully acquired the exclusive rights to Melanotan<br />
and Melanotan Corporation became a major shareholder of the Company. It currently holds a 12.1% share.<br />
<strong>EpiTan</strong> has a strong Intellectual Property position, worldwide marketing rights, and has taken further<br />
steps to ensure commercial exclusivity preventing a likely competitor from gaining any market share.<br />
Australia has the highest rate of skin cancer in the world, and this was the case even before the hole in<br />
the ozone layer became a matter of concern. There is widespread interest and rigorous campaigning to<br />
promote public awareness of the effects of excess exposure to the sun. The geographical location of<br />
<strong>EpiTan</strong> provided the ideal conditions for rapid development of the Company and formed the basis for the<br />
alliance with Melanotan Corporation.<br />
<strong>EpiTan</strong> Strategy<br />
<strong>EpiTan</strong> currently envisages three target markets for Melanotan: prophylactic, therapeutic and cosmetic<br />
applications. Market research has shown that the product has blockbuster potential and could earn<br />
millions of dollars worldwide for the co-development partner, and ultimately for <strong>EpiTan</strong>. Furthermore, a<br />
potentially huge “latent market” may exist – comprising people who would request prescription drugs for<br />
the sake of vanity or to just “feel good” about themselves. This market draws a strong comparison to that<br />
of Botox or Viagra, two products with booming sales. The erectile dysfunction market was valued at<br />
approximately USD 100 million before Viagra reached the market – now the market is soaring at USD 2.2<br />
billion.<br />
<strong>EpiTan</strong>’s current strategy is to find a suitable partner or partners to co-develop, market and sell<br />
Melanotan in the USA, Europe and the Rest of the World, while marketing the product in Australia and<br />
New Zealand on its own. The Company may seek separate partners, each having market dominance in<br />
either one of the regions (Europe, USA, Rest of World). Clearly, the US and European markets have the<br />
greatest potential given their large fair-skinned population.<br />
We understand that the Company is actively progressing talks with prospective pharmaceutical<br />
companies and we expect an announcement at least by Q2/2005. With the new partner, <strong>EpiTan</strong> expects to<br />
receive a one-off signing fee, milestones, financing of remaining clinical studies, marketing and sales<br />
(USA, Europe), and a double digit royalty fee on the marketed product. By any standards, the funding of<br />
clinical trials, marketing and sales is challenging for any biotech company. Supposing <strong>EpiTan</strong> were to be<br />
granted these benefits, it would be just as likely that they would need to give up control of Melanotan at<br />
least partially. In other words, the pharma partner could demand complete control of the clinical trials and<br />
set the timelines for development according to the rest of its pipeline. This is probably a topic of great<br />
discussion with potential pharma partners, and we look forward to the final agreement. In any event,<br />
<strong>EpiTan</strong> expects to maintain the Australia/New Zealand region, and the worldwide IP rights to Melanotan.<br />
The partner/s will receive full exclusive rights to market and distribute Melanotan in other regions.<br />
- 2 -
<strong>EpiTan</strong> plans to take Melanotan into a phase II clinical study as a therapeutic agent to treat PMLE<br />
(Polymorphous Light Eruption), with trials set to begin during this coming European Winter, mainly in<br />
Germany and the UK. It is believed that between 10-20% of the population in the UK, Scandinavia and the<br />
USA suffer from PMLE. Also called sun poisoning, PMLE is a UV-induced skin allergy. As part of the<br />
trial, PMLE will be induced in patients at the outset. After allowing some time for the symptoms to<br />
develop, patients will receive Melanotan (as a sustained-release solid implant), and PMLE induction will<br />
occur again. Pigmentation (levels of melanin) will be measured one or two months later. The efficacy<br />
endpoint will be the reduction in symptoms (skin rash or patient discomfort). It is possible that the trials<br />
progress faster as there is a clearly defined clinical endpoint for this trial. Furthermore, there is a chance<br />
that there will be a quicker regulatory acceptance for this unmet medical need.<br />
Melanotan - the Drug<br />
Melanotan is a synthetic copy of a naturally-occurring hormone, alpha-MSH, which stimulates the<br />
production of melanin in the skin. Melanin is the pigment that gives the skin its dark colour or tanned<br />
appearance. Studies have shown that Melanotan is many times more potent than the naturally occurring<br />
alpha-MSH. Increased levels of melanin protect the body from skin damage as a result of sunburn and<br />
are associated with reduced incidences of skin cancer.<br />
An important issue for the application of Melanotan as a drug for the “mass market” is the right drug<br />
delivery formulation. Originally, Melanotan was developed to be administered as an aqueous<br />
injection, where patients were treated on an “out-patient” basis. This form of treatment was not patientfriendly<br />
as it meant that the patient would need ten daily injections to get an effective Melanotan dose.<br />
<strong>EpiTan</strong> has since explored alternative forms (see chart) of delivering Melanotan and has generated<br />
additional IP protection with these new formulations.<br />
<strong>EpiTan</strong>: Melanothan – Delivery Formulations<br />
Source: <strong>EpiTan</strong><br />
In collaboration with the Southern Research Institute (Alabama, USA), <strong>EpiTan</strong> has successfully developed<br />
a sustained-release solid injectable implant – this is an implant so small and thin that it can be injected<br />
into the patient using a normal blood transfusion needle. Future clinical trials and commercialisation will<br />
focus on this solid implant as the first generation product. The implant will be delivered via a single<br />
injection, delivering the implant under the skin (most likely in the region of the upper arm). The solid<br />
implant will, over 15-20 days, release a steady supply of Melanotan into the blood stream. It is made from<br />
the same material as self-dissolving stitches and does not have to be removed at the end of the treatment.<br />
- 3 -
<strong>EpiTan</strong> is currently conducting a dose-escalation study in Brisbane with this solid implant. We anticipate<br />
that the study will be completed by December 2004, with results expected in early 2005. The next clinical<br />
study, phase II PMLE in Europe, will use this solid implant. A separate phase II study, in sunburn injury,<br />
will be conducted in the USA – this news was published at the AGM held on 1 st October 2004. This trial<br />
will also commence as soon as possible after the dose-escalation results have been established.<br />
In September 2003, <strong>EpiTan</strong> entered into collaboration with pSiMedica <strong>Ltd</strong>., a subsidiary of Australianbased<br />
pSivida to develop a liquid based slow release formulation of Melanotan using pSivida’s BioSilicon<br />
nanotechnology. The microscopic porous structure of BioSilicon, with its “honeycomb” structure, will<br />
allow easy uptake of Melanotan. The slow release formulation will also be administered with a single<br />
liquid injection. <strong>EpiTan</strong> may develop this formulation as a future generation Melanotan product.<br />
In collaboration with Monash University, <strong>EpiTan</strong> has assessed the possibility of an oral formulation of<br />
Melanotan. We imagine that this would be a challenge considering that the drug (which is a peptide)<br />
might lose its potency once in contact with the acidic environment of the stomach. It is worth noting:<br />
there is yet to be a successful oral formulation for Insulin developed.<br />
Presenting a more immediate challenge to <strong>EpiTan</strong> would be getting a topical formulation developed,<br />
which would be more patient-friendly and easier to market and sell that an implant. Following agreements<br />
with several transdermal companies, including CollaGenex Pharmaceutical (USA), Thomas Sköld (Sweden)<br />
and Transdermal Technologies Inc., the development of a topical formulation of Melanotan, i.e. a lotion,<br />
spray or patch is currently underway with pre-clinical studies having just been completed – the results of<br />
which sound promising and further details should be released in the next few weeks. A phase I clinical<br />
trial in humans is planned to start by the end of 2004.<br />
<strong>EpiTan</strong> has prepared an Investigational New Drug (IND) application. Following expert regulatory advice<br />
and discussions with the FDA, <strong>EpiTan</strong> will lodge the IND with the FDA as soon as the dose-escalation<br />
studies are completed to allow clinical trials to begin in the USA. By waiting for the Australian doseescalation<br />
study to complete, <strong>EpiTan</strong> will save by avoiding a duplicate dose study in the USA and will<br />
begin trials in the USA at the phase II level. This phase II is expected to roll into a phase III study and<br />
should commence in 2005.<br />
Following initial talks with the FDA (November 2003), <strong>EpiTan</strong> believes that the most likely indication for<br />
Melanotan will be for the prevention or reduction in skin damage caused by UV exposure in people with<br />
high risk genetically. The slow-release implant is likely to receive approval first as there are similar<br />
products already on the market, such as Zoladex (AstraZeneca) for the treatment of prostate cancer.<br />
Melanotan is expected to be considered a “prescription sunscreen” in the form of this implant.<br />
Melanotan – the Market Potential<br />
A recent independent study by PharmaVentures (Oxford, UK) identified three main markets for<br />
Melanotan. It is worth noting that PharmaVentures was unable to find a comparable product either on the<br />
market or in development. This makes pricing of the drug particularly difficult. Nevertheless,<br />
assumptions were made on the price based on other dermatology products.<br />
The Prophylactic market targets people who do not tan well and are at increased risk of developing skin<br />
cancer. This refers to those who wish to be protected from sunburn and damaging UV radiation, which<br />
increases the risk of skin cancer, particularly people with Fitzpatrick’s Type I and II, with very fair skin.<br />
- 4 -
A word on the Fitzpatrick classification<br />
Melanin determines a person’s skin colour. The quantity of melanin produced by a person’s<br />
melanocytes is determined by genetics. Melanin helps protect the skin from sunburn. For instance,<br />
pale skinned or fair persons have little melanin and dark skinned persons have more melanin.<br />
Fitzpatrick devised a description of skin types known as the Fitzpatrick skin type classification. This<br />
classification denotes 6 different skin types, skin colour, and reaction to sun exposure.<br />
• Type I (very white or freckled) - Always burn<br />
• Type II (white) - Usually burn<br />
• Type III (white to olive) - Sometimes burn<br />
• Type IV (brown) - Rarely burn<br />
• Type V (dark brown) - Very rarely burn<br />
• Type VI (black) - Never burn<br />
Source: www.emedicine.com<br />
Skin cancer is the most prevalent of all cancers. The World Health Organisation claims that there are 2-3<br />
million non-melanoma skin cancers and over 130,000 malignant melanomas occurring globally each year.<br />
Australia has the highest incidence of skin cancer in the world with an average of 750,000 new cases<br />
diagnosed each year. The Australian government spends million of dollars each year trying to increase<br />
awareness of the destructive nature of UV exposure. Accordingly, the market for sun care products<br />
(sunscreens, sun block, etc.) has thrived. In the US, between 1997 and 2000 sun care product sales grew<br />
36% to reach USD 547 million (Source: www.marketresearch.com). In 2002, Australian sales were<br />
approximately AUD 26 million. Research shows that the European market for sun care products was<br />
valued at USD 1.4 billion in 1996.<br />
‣ PharmaVentures estimates the prophylactic market to be approximately USD 1 billion in<br />
annual sales.<br />
The Therapeutic market focuses on people with skin disorders such as PMLE (polymorphous light<br />
eruption), psoriasis, rosacea, solar uticaria, porphyria, vitiligo, and albinism. PharmaVentures has assumed<br />
that the sustained-release solid injectable implant will be the first generation product, to be developed for<br />
the therapeutic, prophylactic and cosmetic markets.<br />
‣ PharmaVentures has identified the therapeutic market to be in excess of USD 500 million in<br />
annual sales.<br />
The Cosmetic market is made up of people who want to look good, with no specific health reasons. These<br />
are mainly the people who go to tanning salons or solariums. The market for tanning salons in the US<br />
alone is estimated at USD 2 billion per annum, with a further USD 100 million spent on self-tanning<br />
products. <strong>EpiTan</strong>’s main focus is to develop an ethical drug to reduce the incidence of skin damage. By<br />
tapping into the “sunless” tanning market, the use of Melanotan will greatly reduce the need to use<br />
tanning salons or the need for sunbathing, thus reducing exposure to harmful UV radiation.<br />
- 5 -
‣ PharmaVentures estimates the cosmetic market to be in excess of USD 3.7 billion in<br />
annual sales.<br />
Aside from this cosmetic market, PharmaVentures has also identified a potential “latent<br />
market”, which describes people who do not currently use any artificial tanning methods.<br />
Melanotan will introduce a new method of feeling and looking better. Ethical products including<br />
Botox injections (“to smooth away wrinkles”) and Viagra (to treat erectile dysfunction) have<br />
given millions of people a better quality of life. Based on these newly created markets,<br />
PharmaVentures believes that Melanotan could target an USD 7 billion potential.<br />
Intellectual Property<br />
Source: <strong>EpiTan</strong><br />
<strong>EpiTan</strong> currently holds 34 granted patents, and is currently lodging patent applications for new IP that<br />
is being generated with the new formulations: slow release implant, lotion, patch, etc. These patents are<br />
expected to offer market protection beyond 2020.<br />
The original patents from the University of Arizona expire in 2008, with Melanotan due to be launched in<br />
2006/2007. However, according to regulatory law (1984 Hatch-Waxman Act), <strong>EpiTan</strong> (or its partners) will<br />
have commercial exclusivity for an additional 5 years in the USA once the product reaches the<br />
market. Similarly, Melanotan will enjoy such exclusivity in Europe for 8 years following its launch.<br />
Source: <strong>EpiTan</strong><br />
- 6 -
Competitors for Melanotan (for the ethical market) have not been identified. To the best of our (and<br />
<strong>EpiTan</strong>’s) knowledge, there are no products in clinical development for this indication. At best, tanning<br />
lotions and tanning salons (solariums) have been identified as competitors. Even if an ethical product was<br />
to begin clinical development now, it would need at least 8 years to reach the market. Melanotan, and<br />
therefore <strong>EpiTan</strong>, remain way ahead of the pack.<br />
Furthermore, <strong>EpiTan</strong> does hold the intellectual property that covers hundreds of analogues of alpha-<br />
MSH (molecules with a similar chemical structure as the naturally occurring hormone) for melanogenesis,<br />
the process of melanin production. These analogues were originally synthesized at the University of<br />
Arizona.<br />
Other Products<br />
In July 2004, <strong>EpiTan</strong> announced that it had acquired three products as part of its strategy to specialise<br />
in prescription dermatology products.<br />
Linotar (eczema) and Exorex (psoriasis) have been acquired from Transdermal <strong>Pharmaceuticals</strong> Australia<br />
Pty <strong>Ltd</strong>. These two products are currently on sale in Australia and are generating roughly AUD 0.5 million<br />
in turnover. <strong>EpiTan</strong> is furthermore awaiting news of Exorex being added to the Pharmaceutical Benefits<br />
Scheme listing in Australia, which would boost sales.<br />
The patents for both products are owned by a South African company (undisclosed). <strong>EpiTan</strong> is currently<br />
finalising the contract, in particular, the supply arrangements. As a result, the Company has not seen<br />
any revenues from these products yet. We expect some news on this deal by the end of 2004. A sales team<br />
for these two products is already in place (out-sourced), so <strong>EpiTan</strong> will not need to invest heavily in<br />
training new staff.<br />
A third product, Zindaclin (acne), has been in-licensed from Strakan <strong>Pharmaceuticals</strong> (UK). Financials<br />
were not disclosed. Zindaclin is awaiting TGA (Therapeutic Goods Administration) registration in<br />
Australia.<br />
All three products will be marketed initially in Australia and New Zealand only and are expected to reach<br />
sales of AUD 3 million for the period 2005/2006.<br />
The Company also revealed at the AGM on 1 st<br />
October 2004, that Management were actively seeking<br />
more in-licensing opportunities for dermatology products, particularly to treat psoriasis, acne and skin<br />
cancer. Further details were not announced. We believe that <strong>EpiTan</strong> is speaking with companies in the UK<br />
and the USA. We would be curious as to whether these potential products are already on the market and<br />
eagerly await news on this. Until now, we believe that <strong>EpiTan</strong> will end this current fiscal year with at<br />
least six dermatology products, including the three mentioned above.<br />
Financials and Shareholder Information<br />
<strong>EpiTan</strong> estimates that the remaining clinical trials (global) and marketing and sales costs for Melanotan<br />
(Australia and New Zealand) should cost roughly AUD 35 million. The Company expects to receive this<br />
funding from the prospective co-development partner. One advantage <strong>EpiTan</strong> enjoys is that costs for<br />
Melanotan clinical studies will be considerably lower than for a typical therapeutic product since the trial<br />
duration is shorter and patients are healthy volunteers, at least for the sunburn injury study. For the<br />
PMLE indication, the high patient numbers (100 million sufferers world wide) point to a fairly swift<br />
recruitment process for the next phase II study (Europe).<br />
- 7 -
<strong>EpiTan</strong> successfully raised AUD 8.9 million in 2003 and a further AUD 8 million in August 2004. <strong>EpiTan</strong>’s<br />
current cash position lies at AUD 10.9 million. In the coming fiscal year, it is expected that this money<br />
will be used as follows:<br />
• cost of running business entity approx. AUD 1.5 million<br />
• Melanotan development costs AUD 7 million<br />
• approx. AUD 0.5 million to be spent on newly acquired products<br />
<strong>EpiTan</strong> does not generate any revenues from product sales as yet. On the other hand, the current cash<br />
burn rate is fairly low at AUD 0.5 million per month.<br />
With <strong>EpiTan</strong> having 125,699,085 ordinary shares, major shareholders include (30 th September 2004):<br />
• Weighton Pty.(Wayne Millen, CEO of <strong>EpiTan</strong>): 14.2%<br />
• Melanotan Corporation Inc.: 12.1%<br />
• FM Fund Management (Absolute Return Europe Fund): 7.1%<br />
In July, <strong>EpiTan</strong> established a Level One American Depositary Receipt (ADR) program and the Bank of<br />
New York was appointed as the depositary bank for this. Each ADR represents 10 ordinary shares of<br />
<strong>EpiTan</strong> as traded on the ASX (Australian Stock Exchange).<br />
<strong>EpiTan</strong> also completed a listing on the Frankfurt Stock Exchange (Xetra) on the 1 st September 2004.<br />
Valuation and Stock Assessment<br />
Based on the figures from the PharmaVentures study as commissioned by <strong>EpiTan</strong>, we have derived a<br />
valuation (Net Present Value or NPV) for Melanotan in the various markets (namely therapeutic,<br />
prophylactic, cosmetic) and for the <strong>EpiTan</strong> stock in general according to a Discounted Cash Flow<br />
valuation. In addition, we have assessed the potential of the product in the Australia/New Zealand (ANZ)<br />
market separately as <strong>EpiTan</strong> will market and sell Melanotan on its own in this region and incur separate<br />
costs.<br />
The table below represents our assumptions and results for the NPV analysis of Melanotan. Perhaps<br />
our most significant assumption in our valuation is that the drug will enter phase III clinical trials and be<br />
launched in the USA, Europe and Australia/New Zealand. Equally important is our assumption that the<br />
Company will secure a pharma partner to finance the remaining clinical trials and the launch, marketing<br />
and sales of Melanotan in the USA and Europe. Our valuation is limited to the first generation product,<br />
the solid injectable sustained release formulation being evaluated in current clinical trials. We have<br />
assigned a 15% royalty rate to <strong>EpiTan</strong>, this being an average industry rate. We keep a conservative view<br />
regarding the market penetration (maximum 15%) – the degree to which Melanotan is able to<br />
penetrate the market will largely depend on the sales and marketing force of the pharma partner.<br />
Following the announcement, we could change our stance on this depending on the partner’s position in<br />
the market. Furthermore, we have factored in a probability of 65% that the product succeeds clinical<br />
tests and enters the market in 2007, based on the assumption that the product is about to enter phase III<br />
trials.<br />
Our discussions with Management combined with our research have led us to the price structure shown in<br />
the table below. Some might argue that it is over-priced at USD 250 per treatment per year – we,<br />
however, believe that it is quite realistic based on current dermatological prescription drugs and the price<br />
of “life style products” such as Botox. As there are no comparable products on the market, it is difficult to<br />
be accurate with the pricing – even PharmaVentures was unable to settle on a number. Once again, we<br />
may have more insight into the pricing once a partner is on board.<br />
- 8 -
We believe that <strong>EpiTan</strong> stands to receive up to USD 20 million in upfront payments and milestones (for<br />
example phase III, approval, and registration) between 2005 and 2007, the market launch. This cash<br />
would be a significant boost for the Company and will help finance <strong>EpiTan</strong>’s plans to scale up sales and<br />
marketing efforts in Australia/New Zealand (ANZ) where they plan to market the drug alone.<br />
Market penetration<br />
Price per<br />
treatment<br />
Net Present Value (NPV)<br />
No. of patients<br />
targeted initially<br />
(worldwide, excl.<br />
MIN MAX (USD) Estimated<br />
Peak Sales<br />
After tax (30%) per Share<br />
ANZ)<br />
(USD) AUD EUR<br />
USD 623<br />
Therapeutic (PMLE) 20.740.000 0.1% 10,0% 250 million (2010) 0,79 0,45<br />
Prophylactic 44.350.000 1,0% 10,0% 200<br />
USD 1.3<br />
billion (2012) 1,39 0,80<br />
Cosmetic 51.220.000 2,0% 15,0% 100<br />
Source: MIDAS Research<br />
USD 997<br />
million (2011) 1,10 0,63<br />
3,28 1,89<br />
We have come to the conclusion that the prophylactic market holds the largest commercial potential for<br />
Melanotan, with a NPV of EUR 0.80 per share (AUD 1.39), based on a retail price of approximately USD<br />
200 (EUR 160) per patient per year. As explained earlier, the prophylactic or preventative market<br />
encompasses those people “who do not tan well and are at increased risk of developing skin cancer”<br />
(according to PharmaVentures). Society is becoming increasingly aware that excess UV exposure is the<br />
primary cause of skin damage and skin cancer. In the USA alone, sales of sun care products (including<br />
sunscreens) amounted to USD 500 million in 2000. Clinical studies to date have proven that Melanotan has<br />
the ability to reduce the skin cell damage (caused by UV exposure) by 50%. More importantly in this<br />
prophylactic market, <strong>EpiTan</strong> needs to develop a topical formulation. In the form of a lotion, gel or patch,<br />
Melanotan would carry an even greater market potential, including of course children.<br />
Following closely behind in terms of commercial value is the therapeutic market, consisting of patients<br />
with UV-associated skin diseases or disorders. In our valuation, we looked at PMLE (a sun allergy) as<br />
<strong>EpiTan</strong> will base future clinical trials on patients with PMLE. Melanotan may initially be marketed as a<br />
treatment for PMLE. In this indication, we assumed that the product will be more expensive, priced at<br />
roughly USD 250 (EUR 200) per patient per year. The resulting NPV of EUR 0.45 (AUD 0.79) per share<br />
shows the therapeutic value of Melanotan compared to the current share price of EUR 0.52 (AUD 0.87).<br />
It is likely that the market penetration in this indication will be small initially as there may be some<br />
barriers to entry. It will take some time for society to warm up to the idea of having this implant,<br />
particularly as it requires a trip to the doctor. Much will depend on changing the mindset of<br />
dermatologists that will be treating and recommending Melanotan as well as marketing efforts. Once<br />
again, the partner’s position in the dermatology market will play a critical role.<br />
The cosmetic market for Melanotan comprises people who wish to have a tan to look good – with no<br />
specific health reasons. In this area, patient numbers could soar based on the solarium industry alone,<br />
worth over USD 2 billion per annum in the USA. Today’s society dictates the importance of striving for a<br />
healthier lifestyle: feeling good is not all – looking good is just as important. We believe <strong>EpiTan</strong> stands the<br />
chance of earning the highest revenues in this market, based on “off-label” use. This would be<br />
particularly true once the second generation product (topical: lotion, gel, patch) entered the market – such<br />
a product would also be attractive to children or teenagers. However, this could take longer to reach the<br />
market than the solid implant. For this reason, we focused on the solid implant making an entry to the<br />
cosmetic market. We prefer to remain cautious and assume a high barrier to entry, and therefore, low<br />
market penetration resulting in a NPV of EUR 0.63 (AUD 1.10) per share at a price of approximately USD<br />
- 9 -
100 (EUR 80) per annual treatment. If the Botox industry is anything to go by, then our caution could be<br />
over-stated.<br />
Within the cosmetic market, PharmaVentures has also identified a “latent” market for applications in those<br />
who do not use artificial tanning products at present – they may later realise that they actually enjoy the<br />
benefits of such a product. We find it much too speculative to valuate this market at this stage, and<br />
therefore, have not put a price to it at this stage.<br />
As <strong>EpiTan</strong> plans to market and sell the product in Australia/New Zealand, we conducted a separate<br />
valuation for Melanotan based on population statistics in this region and the following assumptions:<br />
• a larger market penetration, particularly in the preventative market (up to 17% in 2010), as this<br />
would be a “local product”<br />
• marketing costs at 30% of sales<br />
• manufacturing costs of 5%<br />
• 10% of sales to be paid as fees/royalties to Melanotan Corp. and formulation partners<br />
• launch costs of up to 20% of sales for the first year, amounting to approx. AUD 9 million<br />
The resultant after-tax NPV comes in at EUR 0.91 (AUD 1.57) for all three markets (preventative,<br />
therapeutic and cosmetic) in the ANZ region.<br />
Our DCF-derived fair value for the <strong>EpiTan</strong> Group as a whole is based on the same revenue assumptions<br />
described above in the NPV per product assessment, and included sales in the three markets together with<br />
ANZ revenues. It also included a small revenue stream from the three additional products (Linotar, Exorex,<br />
Zindaclin) collectively making up AUD 3-5 million per year (on average) in sales from 2007 onward.<br />
Management has indicated that they hope to have 6 products altogether in their product portfolio by the<br />
end of their current fiscal year, all of which are either awaiting registration or are currently on the market.<br />
We will receive more of an update in the coming weeks, and have only factored in the three products<br />
mentioned into our model. The DCF further included some general expenses incurred by the Group which<br />
have not been factored into the NPV analysis by product.<br />
The DCF model generates a fair value of EUR 2.13 per share, or AUD 3.63. This value is comparable with<br />
the first method of valuation: the sum of the NPV analysis by product of EUR 1.89 or AUD 3.29, excluding<br />
the ANZ region, and EUR 2.79 or AUD 4.85 including revenues from the ANZ region.<br />
Considering that all of these projections/estimates depend almost entirely on <strong>EpiTan</strong> securing a<br />
partner in the next 6 months, the stock is extremely speculative in our view. At the same time, the upside<br />
potential once a partner is announced seems magnificent. Thus, the current share price of EUR 0.50, or<br />
AUD 0.87 presents a clear buying opportunity as the stock stands to make significant gains in the next 6-<br />
12 months based on what Management has promised to be expected news flow: partner deal, phase III<br />
trials, other formulations, etc. Furthermore, at a price of EUR 0.50, a modest risk could be worth taking.<br />
We initiate coverage of <strong>EpiTan</strong> with a SPECULATIVE BUY recommendation. Our initial target price is<br />
set at EUR 2.00 or AUD 3.50 per share, close to the average of the fair values derived through the DCF<br />
method and the NPV assessment.<br />
- 10 -
Clinical Trials Summary<br />
Clinical phase<br />
Phase I/II<br />
Pharmacokinetic<br />
Initiation/Completion<br />
ended Nov/Dec 2001<br />
Phase II<br />
Sunburn Study<br />
ended Q3/2003<br />
Phase Ib<br />
Implant dose/<br />
Escalation study<br />
Phase II<br />
Genotype<br />
Study<br />
Began Nov 2003<br />
Resumed June 2004<br />
with batch of smaller<br />
Implants<br />
Results Q4/2004<br />
commence late 2004<br />
Phase II<br />
PMLE study<br />
commence late 2004<br />
(European Winter)<br />
Phase III<br />
Sunburn injury<br />
commence mid-2005<br />
Location of the trial<br />
Royal Adelaide Hospital<br />
Royal Prince Alfred Hospital<br />
(Sydney)<br />
Royal Adelaide Hospital<br />
Q-Pharm facility<br />
Clive Berghofer Cancer<br />
Research Centre, Royal<br />
Brisbane Hospital<br />
Queensland Institute of<br />
Medical Research (QIMR)<br />
Germany, UK<br />
Global (Australia, US,<br />
Europe)<br />
Patients/treatment<br />
12 healthy volunteers<br />
Subcutaneous injections for<br />
10 consecutive days<br />
61 Melanotan-treated<br />
20 Placebo<br />
Subcutaneous injections<br />
3 X 10 day treatments (3<br />
months)<br />
Slow-release implant injected<br />
under the skin.<br />
6 patients received implant<br />
Nov 2003<br />
Caucasian subjects with<br />
genetic susceptibility to<br />
sunburn, skin cancer,<br />
skin sun allergy<br />
Patients with Polymorphic<br />
Light<br />
Eruption (PMLE)<br />
Treatment with slow-release<br />
implant<br />
1,000-2,000 healthy<br />
individuals<br />
Objective<br />
Safety and toxicity profile<br />
Efficacy in inducing a tan with<br />
reduced skin cell damage<br />
following controlled UV<br />
exposure<br />
Optimal dosage, safety,<br />
compliance, efficacy of the<br />
implant.<br />
To establish the safety and<br />
degree of tanning in these<br />
genetically predisposed subjects.<br />
Endpoint: reduction in<br />
symptoms (sunburn, rash,<br />
blisters, patches)<br />
Measurement of melanin<br />
increase<br />
Efficacy in inducing melanin<br />
production with reduced skin<br />
cell damage following controlled<br />
UV exposure<br />
Description of trial<br />
Rapid absorption rate, short halflife,<br />
increased levels of melanin in<br />
the skin<br />
Safe and no obvious side effects<br />
Significant increase in melanin.<br />
Melanin density increases 100% in<br />
fair skinned patients.<br />
Amount of skin cell damage,<br />
following controlled UV exposure,<br />
reduced by 50%.<br />
Melanin levels are increased in the<br />
skin by means other than by<br />
damaging the cells with UV<br />
radiation.<br />
In Feb 2004, results showed less<br />
Melanotan needed in implant. New<br />
batch with less drug under<br />
production. Trial was rescheduled<br />
with the smaller implant.<br />
Assays will be developed to detect<br />
melanin receptor deficiencies.<br />
PMLE affects 10-20% of population<br />
in northern latitudes. Study will<br />
determine the individuals to whom<br />
Melanotan can be of most benefit.<br />
<strong>EpiTan</strong> seeks partner to share<br />
costs, co-develop, market and sell<br />
Melanotan.<br />
Launch planned late 2006/early<br />
2007.<br />
Aktienflash
Currency Exchange Rates:<br />
1 AUD = 0.7267 USD = 0.5915 EUR<br />
Source:<br />
<strong>EpiTan</strong> Limited<br />
MIDAS Research GmbH<br />
www.reuters.com<br />
www.maxblue.de<br />
www.marketresearch.com<br />
www.yahoo.com.au<br />
www.bigcharts.com<br />
www.emedicine.com<br />
MIDAS Research Subscription<br />
If you like to receive our research directly by Email please register on our Web Site<br />
http://www.midasresearch.de under „RESEARCH ABO“<br />
Disclaimer<br />
This report is not suited for any individuals resident in any jurisdiction in which access to such reports is regulated by<br />
applicable laws. No investment decision must be based on any aspect of, or statement in, this report. If you are<br />
uncertain if this might apply in your case you should not access and consider this report.<br />
This publication was produced by MIDAS Research GmbH. It represents only a non-binding view of the development of the capital markets as<br />
well as of quoted companies and gives information about the composition and/or change of the model portfolio as composed by MIDAS Research<br />
GmbH. Purpose of the publication is to supply information for the shaping of a personal opinion. The publication does not constitute any<br />
investment advice or recommendation of certain stock exchange transactions and cannot replace professional investment advice. Each reader<br />
remains requested to consult his personal investment advisor to discuss a possible purchase or sale of any of the securities mentioned in the<br />
publication before carrying out any such investment transaction. Data and facts forming basis of this publication have not been separately<br />
verified by MIDAS Research GmbH and MIDAS Research GmbH does not assume any liability for the content of this publication. If this<br />
publication contains opinions as far as the future development of prices of securities or of the business development of enterprises is concerned<br />
these are mere forecasts. The probability that forecast circumstances materialize is subject to substantial risk and can be assured in no way. Any<br />
views expressed in the publication or information about the model portfolio composed by MIDAS Research are only valid for the point in time as<br />
stated on the report and may change at any time without further notice.<br />
MIDAS Research GmbH does not conduct own account trading in shares or related derivatives of the companies discussed in this publication. This<br />
applies also to the authors and other persons connected with MIDAS Research GmbH, if they are familiar with the content of the publication or<br />
could have been familiar due to their business relation with MIDAS Research GmbH, however MIDAS Research GmbH does not assume any<br />
liability to the aforementioned.<br />
If MIDAS Research GmbH directly or indirectly maintains or has directly or indirectly maintained within the last five calendar years Paid<br />
Research Agreements or other Service Agreements with companies mentioned in this report these companies are indicated in the following:<br />
<strong>EpiTan</strong> <strong>Ltd</strong>.<br />
Any reproduction, change or use of this publication without previous written agreement of MIDAS Research GmbH is inadmissible.<br />
EMAIL: info@midas-research.de INTERNET: http://www.midasresearch.de CONTACT: Simone Drepper (responsible) +49 621/430 613 0