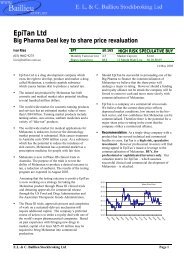
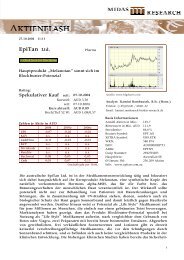
Clinuvel Newsletter May 2011 - Clinuvel Pharmaceuticals
Clinuvel Newsletter May 2011 - Clinuvel Pharmaceuticals
Clinuvel Newsletter May 2011 - Clinuvel Pharmaceuticals
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Clinuvel</strong> Communiqué<br />
MAY <strong>2011</strong><br />
CONTENTS<br />
CEO’S OVERVIEW<br />
CEO’s Overview 1<br />
Confirmatory EPP studies complete in the US & Europe 2<br />
INSPIRE vitiligo program update 2<br />
Guest bloggers and new video releases 3<br />
Share price and financials 3<br />
CLINUVEL REPRESENTATION<br />
AT SCIENTIFIC & FINANCIAL EVENTS<br />
RECENT:<br />
• 29th Annual JP Morgan Healthcare Conference – San Francisco, US<br />
(January 11-14)<br />
• <strong>Clinuvel</strong>’s third Annual Scientific and Clinical Excellence Meeting<br />
(ASCEM III) – Luzern, Switzerland (January 23-24)<br />
• 4th Annual Sachs Associates European Life Science CEO Forum –<br />
Zürich, Switzerland (January 25-26)<br />
• 69th Annual Meeting of the American Academy of Dermatology –<br />
New Orleans, US (February 4-8)<br />
• BIO-Europe Spring <strong>2011</strong> – Milan, Italy (March 14-16)<br />
• Porphyrias and Porphyrins <strong>2011</strong> – Cardiff, Wales (April 11-14)<br />
• Generational Dermatology Summit – New York, US (April 29-<strong>May</strong> 1)<br />
At a final stage of preparing <strong>Clinuvel</strong>’s regulatory filing in Europe,<br />
many activities take place in-house to optimise a successful outcome.<br />
To arrive at this stage is truly remarkable as we took less than five<br />
years of development since starting the program in erythropoietic<br />
protoporphyria (EPP).<br />
In this newsletter, I shall take you back to the sequence of events the<br />
past quarter.<br />
We welcomed Eli Ishag as new Board member, providing the<br />
Board additional commercial input at this stage of preparing the<br />
commercialization of SCENESSE® (afamelanotide).<br />
In January we organized the third ACSEM in Luzern, where world’s<br />
leading clinicians, scientists and experts in the fields of alpha-MSH,<br />
cell biology and dermal carcinogenesis attended. This meeting<br />
has become a forum of excellence where the latest developments<br />
and insights are being discussed and presented. Additionally, our<br />
scientific teams exchange views on the application of afamelanotide<br />
in the clinic. It is essential to <strong>Clinuvel</strong>’s existence to obtain regular<br />
and unbiased feedback on the use of SCENESSE®.<br />
This quarter we learned about the progressive adoption of<br />
SCENESSE® by Italian EPP patients, whereby we recorded for the<br />
first time sales of the drug in a European country. Feedback is<br />
excellent, and patients and physicians report an increase demand for<br />
SCENESSE®. Italy serves as a good benchmark for years to come.<br />
UPCOMING:<br />
• World Congress of Dermatology – Seoul, Korea (<strong>May</strong> 24-29)<br />
• World Orphan Drug Summit – Frankfurt, Germany (<strong>May</strong> 31-June 1)<br />
• XXIst International Pigment Cell Conference – Bordeaux, France<br />
(September 21-24)<br />
• 20th Congress of the European Association of Dermatology and<br />
Venereology (EADV) – Lisbon, Portugal (October 20-24)<br />
After discussions with FDA in January and February, we altered<br />
the vitiligo program (INSPIRE) with separate European and<br />
US trial protocols (CUV101 and CUV102). The US trial, which<br />
commenced recruitment last week, will see patients administered<br />
four afamelanotide doses to ‘active’ cohorts over six months. EU<br />
centers, awaiting Ethics approval for a further amendment, will<br />
administer six afamelanotide doses over six months. The combination<br />
of SCENESSE® with narrowband UVB has set high expectations<br />
(Continued on Page 2)
<strong>Clinuvel</strong> Communiqué <strong>May</strong> <strong>2011</strong><br />
among the medical and patient community as witnessed by the high<br />
online demand for information and we will provide further updates as<br />
approvals warrant.<br />
The highlight of the past weeks was undoubtedly our submission of<br />
documentation to the European Medicines Agency (EMA) and<br />
its respective committee COMP. Early <strong>May</strong> <strong>Clinuvel</strong> was invited to<br />
attend a meeting in London to discuss the dossier on SCENESSE® in<br />
the use of EPP. As you will know by now, the outcome was positive<br />
and <strong>Clinuvel</strong> was given positive feedback on the quality of its dossier,<br />
whereby the results of our confirmatory EPP studies (CUV029 and<br />
CUV030) would be the final determinants of efficacy. After five<br />
years of starting this program, the outcome summarises a collective<br />
achievement from the <strong>Clinuvel</strong> teams across Australia, Europe and the<br />
US. At times, and much to my personal frustration, to have to await<br />
lengthy regulatory procedures the clock seemed to be ticking slower<br />
than desired, however against industry benchmarks to be able to file<br />
within five years of commencement is a great moment. Pending the<br />
outcome of these clinical results, <strong>Clinuvel</strong> aims to lodge its European<br />
filing before January 1 2012.<br />
We are now awaiting the final results of CUV029 and CUV030<br />
anticipated the next weeks to months. This will ultimately show us<br />
whether SCENESSE® is effective in EU and US patients to prevent or<br />
reduce their symptoms. Additionally, we will release PLE (CUV032)<br />
results which will serve as safety data to our filing. An interim<br />
analysis on CUV011, actinic keratosis and squamous cell carcinoma in<br />
immunocompromised transplant patients, is under way and expected<br />
this year.<br />
The demand for our drug is high. We are all aware of this and our<br />
relentless commitment is to demonstrate that SCENESSE® deserves<br />
a position in the pharmaceutical list of reimbursable drugs. Clinical<br />
response has been excellent; the challenge remains to obtain approval<br />
in Europe first, followed by the US (FDA). <strong>Clinuvel</strong> has entered a final<br />
stage, a unique position where not many companies have arrived.<br />
I thank you for your patience and ongoing support, drug<br />
development requires a long term view from all of us, but will be<br />
rewarding for patients and investors.<br />
Philippe Wolgen<br />
CONFIRMATORY EPP STUDIES COMPLETE IN THE US &<br />
EUROPE<br />
Results from CUV029 and CUV030 are due in the coming months<br />
and the company expects them to add to the first dossier for a<br />
marketing authorisation application with the European Medicines<br />
Agency. Discussions are still ongoing with the FDA to set the next<br />
steps for <strong>Clinuvel</strong>’s US EPP program.<br />
INSPIRE VITILIGO PROGRAM UPDATE<br />
Vitiligo is a disease which causes white or off-white depigmented<br />
skin lesions to appear on different parts of the body due to a loss of<br />
melanin (pigment) production. This disorder may spread over time<br />
and cause patients significant psychological and emotional distress.<br />
In March, <strong>Clinuvel</strong> announced that the US Food and Drug<br />
Administration (FDA) and the Italian Medicines Agency (AIFA)<br />
had allowed the company to commence a pilot Phase II study of<br />
SCENESSE® (afamelanotide) in patients diagnosed with nonsegmental<br />
vitiligo, the most common form of the disease which affects over 45<br />
million individuals globally.<br />
The International SCENESSE® Pilot Repigmentation Evaluation<br />
(INSPIRE) program will evaluate the efficacy of SCENESSE® as<br />
a combination therapy with narrowband ultraviolet B (NB-UVB)<br />
phototherapy compared to NB-UVB alone. The goal of the trial is to<br />
determine whether SCENESSE® will reduce the total dose of radiation<br />
(NB-UVB) and the time required to reactivate skin pigment producing<br />
cells in vitiliginous lesions.<br />
Following further discussions with regulators, <strong>Clinuvel</strong> took the<br />
decision to run parallel protocols in Europe and the US (CUV101 and<br />
CUV102 respectively). These protocols will provide results on the<br />
safety and efficacy of the drug when given every 28 days alongside<br />
six months’ NB-UVB phototherapy (CUV101) and when dosing<br />
commences two months into a six month NB-UVB treatment regime<br />
(CUV102). Each study site will recruit approximately 20 patients, with<br />
50% of the patients treated with NB-UVB alone, and 50% treated in<br />
combination with SCENESSE®.<br />
Further amendments have been made to broaden the potential<br />
patient population, a reflection of feedback from the patient and<br />
medical communities asking for the inclusion of patients who have<br />
undergone previous treatment for their vitiligo. These amendments<br />
have lead to delays in the INSPIRE program, but are expected to<br />
generate quality clinical data from which we can evaluate SCENESSE’s<br />
potential as a vitiligo therapy.<br />
The company announced in February that all patient visits for the<br />
first Phase II study of SCENESSE® (afamelanotide) to be conducted<br />
in the US had been completed. The study (CUV030) – a six month,<br />
randomised, placebo-controlled trial – was designed to further<br />
evaluate the safety and efficacy of SCENESSE® in reducing the<br />
number and severity of phototoxic skin reactions in patients with the<br />
rare light intolerance disorder erythropoietic protoporphyria (EPP).<br />
The protocol of CUV030 closely followed that of a confirmatory<br />
Phase III study being conducted in European sites (CUV029). All<br />
patient visits for the CUV030 and CUV029 have completed and data<br />
collection and management is under way.<br />
In the last week, our first US site has commenced recruitment for<br />
CUV102. Two further US sites are expected to commence recruitment<br />
shortly. European protocol amendments are currently being evaluated<br />
by the relevant regulatory authorities with the first patients expected<br />
to commence across the three sites (France, Italyand Switzerland) in<br />
June.<br />
For more information on <strong>Clinuvel</strong>’s vitiligo program, log onto http://<br />
www.clinuvel.com/vitiligo<br />
Page 2
<strong>Clinuvel</strong> Communiqué <strong>May</strong> <strong>2011</strong><br />
GUEST BLOGGERS AND NEW VIDEO RELEASES<br />
Over recent months the team has invited several individuals to<br />
share their experiences of erythropoietic protoporphyria (EPP),<br />
polymorphous light eruption (PLE) and vitiligo on the company’s blog.<br />
You can read all of these posts at http://www.clinuvel.com/en/blog/<br />
guest/<br />
To improve awareness of EPP and vitiligo online, the company has<br />
released two new videos since April.<br />
The first Burning in the Shadows is the latest in a series of videos<br />
featuring Mikey, an Australian man with EPP. Mikey explains there is<br />
no escape from light when outside for those with EPP and that even<br />
in the shadows he has been in agony from reflected light alone.<br />
The second video, Understanding vitiligo, explores established and<br />
recent theories that explain what causes vitiligo, a disease which is<br />
still not fully understood by the medical community.<br />
To view these videos, log on to the <strong>Clinuvel</strong> website or our Youtube<br />
channel at http://www.youtube.com/photoprotection.<br />
SHARE PRICE AND FINANCIALS<br />
Shares on issue<br />
30,381,706<br />
<strong>Clinuvel</strong> is listed on XETRA (UR9) and<br />
has a level 1 ADR (CLVLY)<br />
Average monthly cash burn Jan-Mar ‘11<br />