ACCORD SOP INDEX PAGE
ACCORD SOP INDEX PAGE
ACCORD SOP INDEX PAGE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>ACCORD</strong> <strong>SOP</strong> <strong>INDEX</strong> <strong>PAGE</strong><br />
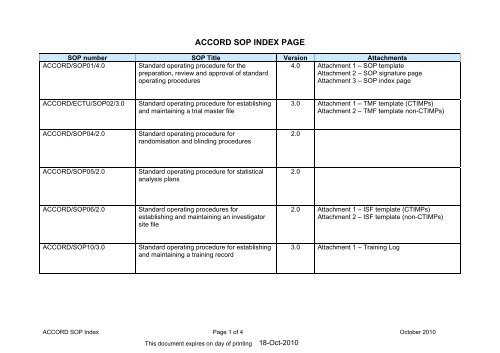
<strong>SOP</strong> number <strong>SOP</strong> Title Version Attachments<br />
<strong>ACCORD</strong>/<strong>SOP</strong>01/4.0<br />
Standard operating procedure for the<br />
preparation, review and approval of standard<br />
operating procedures<br />
4.0 Attachment 1 – <strong>SOP</strong> template<br />
Attachment 2 – <strong>SOP</strong> signature page<br />
Attachment 3 – <strong>SOP</strong> index page<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>02/3.0<br />
Standard operating procedure for establishing<br />
and maintaining a trial master file<br />
3.0 Attachment 1 – TMF template (CTIMPs)<br />
Attachment 2 – TMF template non-CTIMPs)<br />
<strong>ACCORD</strong>/<strong>SOP</strong>04/2.0<br />
Standard operating procedure for<br />
randomisation and blinding procedures<br />
2.0<br />
<strong>ACCORD</strong>/<strong>SOP</strong>05/2.0<br />
Standard operating procedure for statistical<br />
analysis plans<br />
2.0<br />
<strong>ACCORD</strong>/<strong>SOP</strong>06/2.0<br />
Standard operating procedures for<br />
establishing and maintaining an investigator<br />
site file<br />
2.0 Attachment 1 – ISF template (CTIMPs)<br />
Attachment 2 – ISF template (non-CTIMPs)<br />
<strong>ACCORD</strong>/<strong>SOP</strong>10/3.0<br />
Standard operating procedure for establishing<br />
and maintaining a training record<br />
3.0 Attachment 1 – Training Log<br />
<strong>ACCORD</strong> <strong>SOP</strong> Index Page 1 of 4 October 2010
<strong>SOP</strong> number <strong>SOP</strong> Title Version Attachments<br />
<strong>ACCORD</strong>/<strong>SOP</strong>11/5.0<br />
Standard operating procedure for identifying,<br />
recording and reporting adverse events for<br />
clinical trials of investigational medicinal<br />
products<br />
5.0 Attachment 1 – SAE report template<br />
Attachment 2 – SUSAR report template<br />
Attachment 3 – Fax cover sheet<br />
Attachment 4 – <strong>ACCORD</strong> fax receipt form<br />
Attachment 5 – MREC safety report form<br />
Attachment 6 – Pregnancy notification<br />
form<br />
Attachment 7 – AE reporting flowchart<br />
Attachment 8 – Identifying AEs flowchart<br />
<strong>ACCORD</strong>/<strong>SOP</strong>12/2.0<br />
<strong>ACCORD</strong>/<strong>SOP</strong>13/3.0<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>14/2.0<br />
<strong>ACCORD</strong>/<strong>SOP</strong>15/2.0<br />
Standard operating procedure for identifying,<br />
recording and reporting adverse events for<br />
research other than clinical trials of<br />
investigational medicinal products<br />
Standard operating procedure for archiving<br />
clinical research data<br />
Standard operating procedure for writing a<br />
protocol to good clinical practice<br />
Standard operating procedure for preparing<br />
and submitting progress and safety reports<br />
2.0 Attachment 1 – REC reporting form<br />
3.0<br />
2.0 Attachment 1 – Protocol template for CTIMPs<br />
Attachment 2 – Protocol template for CTIMPs<br />
with explanatory text<br />
2.0 Attachment 1 – Annual Progress Report (non-<br />
CTIMPs)<br />
Attachment 2 – Annual Progress Report<br />
(CTIMPs)<br />
Attachment 3 – <strong>ACCORD</strong> annual safety report<br />
template<br />
Attachment 4 – <strong>ACCORD</strong> annual safety report<br />
template (explanatory text)<br />
Attachment 5 – NRES safety report covering<br />
form<br />
Attachment 6 – NRES Declaration of the End of<br />
<strong>ACCORD</strong> <strong>SOP</strong> Index Page 2 of 4 October 2010
<strong>SOP</strong> number <strong>SOP</strong> Title Version Attachments<br />
a Study<br />
Attachment 7 - Declaration of the End of Trial<br />
Notification Form<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>16/3.0<br />
<strong>ACCORD</strong>/<strong>SOP</strong>18/2.0<br />
<strong>ACCORD</strong>/<strong>SOP</strong>/19/2.0<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>21/2.0<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>22/2.0<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>23/2.0<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>24/2.0<br />
Standard operating procedure for study<br />
closure<br />
Standard operating procedure for study start<br />
up<br />
Standard operating procedure for recording<br />
and managing research study data on case<br />
report forms and other source documents<br />
Standard operating procedure for validation<br />
and functional testing of study databases<br />
Standard operating procedure for data<br />
collection and handling on study databases<br />
Standard operating procedure for study<br />
database security measures<br />
Standard operating procedure for alternative<br />
data recording methods<br />
3.0 Attachment 1 – End of trial notification form<br />
Attachment 2 – Declaration of the end of a<br />
study form<br />
2.0<br />
2.0<br />
2.0<br />
2.0<br />
2.0<br />
2.0<br />
<strong>ACCORD</strong> <strong>SOP</strong> Index Page 3 of 4 October 2010
<strong>SOP</strong> number <strong>SOP</strong> Title Version Attachments<br />
<strong>ACCORD</strong>/<strong>SOP</strong>25/2.0<br />
Standard operating procedure for escalation<br />
and notification of serious breaches of GCP or<br />
the trial protocol<br />
2.0 Attachment 1 – MHRA Notification of<br />
Serious Breaches of GCP or Trial Protocol<br />
Form<br />
Attachment 2 – Notification examples<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>39/1.0<br />
<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>41/1.0<br />
<strong>ACCORD</strong>/<strong>SOP</strong>53/2.0<br />
Standard operating procedure for document<br />
version control<br />
Standard operating procedure for set-up of<br />
work plans<br />
Standard operating procedure for<br />
management of protocol deviations, violations<br />
and urgent safety measures<br />
1.0<br />
1.0 Attachment 1 – work plan template<br />
2.0 Attachment 1 – <strong>ACCORD</strong> template log<br />
of Protocol Deviations, Violations,<br />
Serious Breaches and Urgent Safety Measures<br />
Attachment 2 - <strong>ACCORD</strong> template Protocol<br />
Violation form<br />
Attachment 3 – Notification of Amendment form<br />
<strong>ACCORD</strong> <strong>SOP</strong> Index Page 4 of 4 October 2010