Penumbra Scientific Session News - Penumbra, Inc.
Penumbra Scientific Session News - Penumbra, Inc.
Penumbra Scientific Session News - Penumbra, Inc.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Penumbra</strong> <strong>Scientific</strong> <strong>Session</strong> <strong>News</strong><br />
SNIS 2012<br />
Europe Edition<br />
San Diego, CA<br />
July 2012 | Volume 4<br />
ISCHEMIC<br />
ACCESS<br />
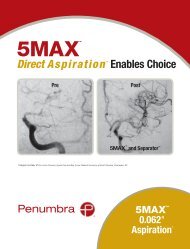
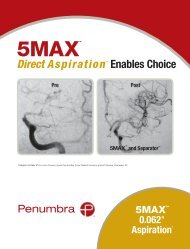
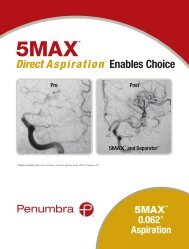
<strong>Penumbra</strong> System ®<br />
MAX <br />
Simplifies Stroke Procedures<br />
Dr. Sam Zaidat, Director of the Neurointerventional Program at the Medical College of<br />
Wisconsin reported the addition of <strong>Penumbra</strong> MAX Reperfusion Catheters (3MAX, 4MAX,<br />
and 5MAX) to the Therapy Trial protocol 1 . Dr. Zaidat noted the new, advanced polymer and<br />
Nitinol coil reinforcement design have optimized the trackability of these catheters, allowing<br />
the 4MAX and 3MAX specifically to be<br />
delivered over a 0.014” wire alone. In one of the<br />
cases he presented, a 3MAX easily tracked into a fetal PComm<br />
and quickly removed thrombus that had been embolized earlier<br />
by a commercially available stent retriever. The new MAX line of<br />
Reperfusion Catheters offers the complete range of therapeutic options<br />
to interventionalists on a simplified, fully compatible aspiration platform.<br />
Hemorrhagic<br />
<strong>Penumbra</strong> Coil 400 have Higher Occlusion Rates<br />
at 6 Months than Conventional Coils<br />
Dr. Aman Patel, Professor, Neurosurgery/Radiology, Mt. Sinai School of Medicine, et. al.<br />
presented a single center, retrospective case review of 111 aneurysms, in the abstract,<br />
Aneurysm Embolization Treatment Efficiency: Comparing the <strong>Penumbra</strong> Coil 400 System to<br />
Conventional Coils 2 . The PC 400 cases had a greater percentage of Class I Raymond Scale<br />
occlusion (88.9%), despite statistically significant greater aneurysm volume, compared to<br />
controls (61.4%).“Our data confirms that when compared to conventional coils, the PC 400 is<br />
indeed more efficient in the embolization of cerebral aneurysms,” the study concluded.<br />
<strong>Penumbra</strong> Redefines<br />
Endovascular Access with<br />
New PX 400 Coil Delivery<br />
Microcatheter<br />
In the abstract, Redefining Endovascular<br />
Access for Treatment of Intracranial<br />
Aneurysms, Dr. Thomas Grobelny, Director,<br />
Neurosciences Institute, Advocate Christ<br />
Medical Center, Oak Lawn, IL, noted that<br />
the <strong>Penumbra</strong> PX 400 microcatheter<br />
had navigation, aneurysm selection and<br />
coil delivery characteristics similar to<br />
conventional microcatheters. 3<br />
He concluded that the advanced<br />
technology and material engineering<br />
used redefines microcatheter size<br />
limits for treatment of aneurysms<br />
and may enable a significant<br />
advancement not<br />
previously possible<br />
with conventional<br />
coil treatment.<br />
PX 400<br />
microcatheter<br />
PC 400<br />
(.020") (n=16)<br />
Conventional Coils<br />
(.010"–.015") (n=95)<br />
Aneurysm Volume 204.3 a 154.5<br />
Mean # Coils Used 3.9 5.5<br />
Mean Procedure Time (min) 46.7 58.8<br />
Mean Packing Density (%) 36.2 b 27.5<br />
Procedural Events 0 0<br />
Raymond Scale-Class I Occlusion at 6<br />
Months Post-procedure (%)<br />
Comparison to Immediate Post-treatment:<br />
Better / Same / Worse (%)<br />
88.9<br />
(n=9)<br />
61.4<br />
(n=44)<br />
66.7 / 33.3 / 0 36.4 / 52.2 / 11.4<br />
<strong>Penumbra</strong> Coil 400<br />
Complex Extra Soft<br />
Statistically Significant: a P < 0.05 b P < 0.005 (Nonparametric Wilcoxon Ranked Text)
ISCHEMIC<br />
Maximizing Treatment Effect<br />
in the THERAPY Trial<br />
The Interventional Management of Stroke<br />
III (IMSIII) Trial was halted earlier this year<br />
because the trial investigators did not<br />
believe it would show statistically superior<br />
results for stroke intervention over<br />
intravenous drug use alone.<br />
THERAPY, a randomized control study<br />
being conducted by <strong>Penumbra</strong>,<br />
<strong>Inc</strong>. continues the mission to study<br />
interventional stroke treatment outcomes<br />
begun by IMSIII. Dr. J Mocco, Associate<br />
Professor of Neurological Surgery,<br />
Radiology, and Radiological Sciences<br />
at the Vanderbilt University Medical<br />
Center, emphasized that the goal of any<br />
randomized control trial design is to<br />
demonstrate a treatment effect between<br />
the control arm and interventional arm 4 .<br />
Clinical evidence from the START Trial<br />
showed that patients selected for<br />
small core infarct volume reported the<br />
best outcomes. However, Dr. Mocco<br />
explained that core infarct volume makes<br />
a poor patient selection criterion for a<br />
randomized control trial because it will<br />
improve outcomes in both the control and<br />
interventional treatment arms and fail to<br />
demonstrate a large treatment effect.<br />
THERAPY applies a strict patient selection<br />
criterion (length of clot burden in mm) to<br />
select patients who will respond poorly<br />
to IV-tPA alone in the control arm but<br />
who will most benefit from a combined<br />
approach using IV-tPA and interventional<br />
clot removal with the <strong>Penumbra</strong> System<br />
in the investigational arm. If successful,<br />
these patient selection criteria could be<br />
widely applied in the industry and improve<br />
the standard of care for ischemic<br />
stroke patients.<br />
Dr. Johannes Weber from<br />
St. Gallen, Switzerland, Presents<br />
Early Experience with the<br />
<strong>Penumbra</strong> 3D<br />
Dr. Johannes Weber, Director of Diagnostic<br />
and Interventional Neuroradiology,<br />
University Hospital St. Gallen, St. Gallen,<br />
Switzerland reported his early experience<br />
with <strong>Penumbra</strong> 3D 5 . Dr. Weber remarked<br />
that the unique intraluminal 3D chambers<br />
make the 3D the next innovation in clot<br />
extraction devices.<br />
In the first fourteen patients treated with<br />
the 3D device at St. Gallen, Dr. Weber<br />
reported 94% revascularization to TICI 2b<br />
or above, with no observed vasospasm<br />
or embolization of new territory (ENT).<br />
He noted the advantages of combining<br />
lesional aspiration with the 3D, which<br />
include reducing the risk of losing<br />
thrombus to a new territory while<br />
providing a multi-modal approach to clot<br />
removal. The 3D device is currently being<br />
investigated in an FDA approved IDE trial<br />
in the US.<br />
START Trial Confirms Favorable<br />
<strong>Penumbra</strong> SystemOutcomes<br />
with 48% of Patients Living<br />
Independently at 90 Days<br />
A presentation on the interim results of<br />
the START Trial by Dr. Don Frei, Director<br />
of Neurointerventional Surgery, Radiology<br />
Imaging Associates/Swedish Medical<br />
Center in Denver, CO, and a Principal<br />
Investigator of the START Trial, opened<br />
the scientific session on the first day of the<br />
SNIS annual meeting 6 . A panel of reviewers<br />
voted the START Trial as “best science”<br />
of the meeting.<br />
The START Trial, a prospective, multicenter<br />
core-lab adjudicated study, reports<br />
48% (37/77) of patients achieved<br />
mRS ≤ 2 at 90 days after treatment<br />
with the <strong>Penumbra</strong> System ® . TIMI 2–3<br />
revascularization was achieved in 86% of<br />
patients. When patients were selected by<br />
ASPECTS on CTA source images, the rate<br />
of good outcomes (mRS 0–2 at 90 days)<br />
was 64.3% in the ASPECTS 8–10 group.<br />
Mean age for the START dataset was 66<br />
with a mean NIHSS of 19.4.<br />
Hemorrhagic<br />
High Volume Center Demonstrates<br />
Wide Use of <strong>Penumbra</strong> Coil 400 <br />
In their abstract, Clinical Experience and<br />
Lessons Learned with the <strong>Penumbra</strong><br />
PC 400 Large Volume Coil: Improving<br />
the Treatment of Both Large and Small<br />
Aneurysms, Drs. Blaise Baxter and Steven<br />
Quarfordt of Erlanger Medical Center,<br />
Chatanooga, TN, concluded that the<br />
“softness by design of this large volume<br />
coil combined with the improved body of<br />
the deployment catheter has markedly<br />
improved the treatment of both large and<br />
small aneurysms.” 7<br />
“Follow-up data suggest that the use of<br />
the coils produced durable occlusions,”<br />
concluded the investigators.<br />
SOURCES<br />
1. Zaidat O. THERAPY Trial: New Devices Update. Symposium<br />
presentation at: 10th Annual Meeting of the Society of<br />
Neurointerventional Surgery; July 23-26, 2012; San Diego,<br />
CA. USA.<br />
2. Patel A, Kamath A, Ploykarpou M, Mascitelli J, Patel<br />
A, Moyle H. Aneurysm Embolization Treatment Efficiency:<br />
Comparing the <strong>Penumbra</strong> Coil 400TM System to Conventional<br />
Coils. Paper presented at: 10th Annual Meeting of the<br />
Society of Neurointerventional Surgery; July 23-26, 2012;<br />
San Diego, CA. USA.<br />
3. Grobelny T. Redefining Endovascular Access for Treatment<br />
of Intracranial Aneurysms. Poster presented at: 10th Annual<br />
Meeting of the Society of Neurointerventional Surgery; July<br />
23-26, 2012; San Diego, CA. USA.<br />
4. Mocco J. THERAPY Trial: Design and Rationale—The<br />
Randomized, Concurrent Controlled Trial to Assess the<br />
<strong>Penumbra</strong> System’s Safety and Effectiveness in the Treatment<br />
of Acute Stroke. Symposium presentation at: 10th<br />
Annual Meeting of the Society of Neurointerventional<br />
Surgery; July 23-26, 2012; San Diego, CA. USA<br />
5. Weber J. Early European Experience with the <strong>Penumbra</strong><br />
3D. Symposium presentation at: 10th Annual Meeting of the<br />
Society of Neurointerventional Surgery; July 23-26, 2012;<br />
San Diego, CA. USA<br />
6. Frei D, Yoo A, Heck D, et al. Pre-treatment CTA Aspects<br />
as a Predictor of Clinical Outcome in Endovascular Stroke<br />
Therapy (EVT): Results from the <strong>Penumbra</strong> START Trial.<br />
Paper presented at: 10th Annual Meeting of the Society of<br />
Neurointerventional Surgery; July 23-26, 2012; San Diego,<br />
CA. USA.<br />
7. Baxter B, Quarfordt S. Clinical Experience and Lessons<br />
Learned with the <strong>Penumbra</strong> PC 400 Large Volume Coil:<br />
Improving the Treatment of Both Large and Small<br />
Aneurysms. Poster presented at: 10th Annual Meeting of<br />
the Society of Neurointerventional Surgery; July 23-26,<br />
2012; San Diego, CA. USA.<br />
www.penumbrainc.com<br />
Copyright ©2012 <strong>Penumbra</strong>, <strong>Inc</strong>. All rights reserved. The <strong>Penumbra</strong> logo, <strong>Penumbra</strong> System, <strong>Penumbra</strong> System MAX, <strong>Penumbra</strong> Coil 400 and <strong>Penumbra</strong> 3D are registered trademarks<br />
or trademarks of <strong>Penumbra</strong>, <strong>Inc</strong>. in the USA and other countries. All other brands and product names are registered trademarks or trademarks of their respective owners. CO LI 5654, Rev. A 08/12 OUS