5MAX - Penumbra, Inc.
5MAX - Penumbra, Inc.
5MAX - Penumbra, Inc.
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
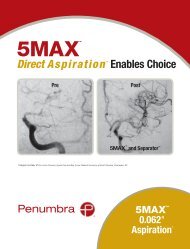
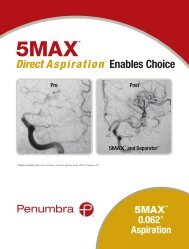
<strong>5MAX</strong>Direct Aspiration Enables ChoicePrePost<strong>5MAX</strong> and Separator Images courtesy of Drs. Imran Chaudry, Aquilla Turk and Ray Turner, Medical University of South Carolina, Charleston, SC<strong>5MAX</strong> 0.062"1Aspiration
Rate of Embolization to New Territory (ENT) 29%7%0.3%<strong>Penumbra</strong> System ®Trevo ENTSolitaire 3 4 5,6
TMMAXTechnology Optimizes Aspiration+83%+200%<strong>5MAX</strong>+83%4MAX0540323MAX041Flow RateData on fi le. Flow rates of 3MAX, 4MAX and <strong>5MAX</strong> using MAX Pump and MAX Aspiration Tubing vs. 032, 041 and 054 using current pump and PST1.TMNew MAXPump• Delivers close to pure vacuum*• Large 0.088" ID reinforced tubingfor efficient flow• Simplified setup• QuietUse of MAX Pump with <strong>Penumbra</strong> System® is indicated for 20 inHg of vacuum.*Pure vacuum is 29.75 inHg
TM<strong>5MAX</strong>TMDirect AspirationEnables Choice• Direct Aspiration TMis applied to the site of occlusion• <strong>5MAX</strong> reduces risk of embolization to new territory (ENT)• Maintains access for adjunctive devices<strong>5MAX</strong> Separator<strong>5MAX</strong> 0.025" IDVelocity <strong>Penumbra</strong> – Stroke Market Leader<strong>5MAX</strong> PositionNeurovascularEmbolectomyDevices1 st <strong>Penumbra</strong>2 nd Covidien3 rd StrykerSource: Millenium Research Group, published Dec. 2012© 2013 Millennium Research Group, <strong>Inc</strong>. All rights reserved. Reproduction, distribution,transmission or publication is prohibited. Reprinted with permission.
TM<strong>5MAX</strong>TMDirect AspirationEnables Choice• Direct Aspiration TMis applied to the site of occlusion• <strong>5MAX</strong> reduces risk of embolization to new territory (ENT)• Maintains access for adjunctive devices<strong>5MAX</strong> Separator<strong>5MAX</strong> 0.025" IDVelocity <strong>Penumbra</strong> – Stroke Market Leader<strong>5MAX</strong> PositionNeurovascularEmbolectomyDevices1 st <strong>Penumbra</strong>2 nd Covidien3 rd StrykerSource: Millenium Research Group, published Dec. 2012© 2013 Millennium Research Group, <strong>Inc</strong>. All rights reserved. Reproduction, distribution,transmission or publication is prohibited. Reprinted with permission.
<strong>Penumbra</strong> System ® MAX Ordering InformationCatalogNumberDescriptionProximalODDistalODProximalIDDistalIDLengthCompatible<strong>Penumbra</strong> DevicesReperfusion Catheters (Working)3MAXC 3MAX Reperfusion Catheter 4.7 F (0.062 in) 3.8 F 0.043 in 0.035 in 153 cm 3MAX Separator TM4MAXC 4MAX Reperfusion Catheter 6.0 F (0.080 in) 4.3 F 0.064 in 0.041 in 139 cm 4MAX SeparatorPSC054 <strong>5MAX</strong> Reperfusion Catheter 6.0 F (0.080 in) 5.0 F 0.064 in 0.054 in 132 cm <strong>5MAX</strong> SeparatorSeparators (Total)3MAXS 3MAX Separator n/a 0.030 in n/a n/a 190 cm 3MAX Reperfusion CatheterPSF041 4MAX Separator n/a 0.035 in n/a n/a 175 cm 4MAX Reperfusion CatheterPSF054 <strong>5MAX</strong> Separator n/a 0.045 in n/a n/a 175 cm <strong>5MAX</strong> Reperfusion CatheterDelivery Microcatheter (Working)VEL160STR Velocity Microcatheter 2.95 F (0.038 in) 2.6 F (0.034 in) 0.025 in 0.025 in 160 cm 4MAX Reperfusion Catheter<strong>5MAX</strong> Reperfusion CatheterAspiration AccessoriesPMX110 MAX Pump MAX CanisterPAPS2 MAX Canister MAX PumpPST2 MAX Aspiration Tubing All Reperfusion CathetersNeuron MAX 6F 088 Lumen Long Sheath Ordering InformationCatalogNumberDescriptionTipShapeWorkingLengthDistal FlexibleZoneOuter Diameter*Proximal /DistalInnerDiameterWireCompatibility(Crosscut Valve, RHV and Dilator <strong>Inc</strong>luded)PNML6F088804 6F 088 Neuron MAX Long Sheath, 80/4 Straight 80 cm 4 cm 8F / 8F .088" .035 / .038”PNML 6F088804M 6F 088 Neuron MAX Long Sheath, 80/4 MP 80 cm 4 cm 8F / 8F .088" .035 / .038”PNML6F088904 6F 088 Neuron MAX Long Sheath, 90/4 Straight 90 cm 4 cm 8F / 8F .088" .035 / .038”PNML6F088904M 6F 088 Neuron MAX Long Sheath, 90/4 MP 90 cm 4 cm 8F / 8F .088" .035 / .038"1. Data on fi le, <strong>Penumbra</strong>, <strong>Inc</strong>.2. Fiehler J, Söderman M, Turjman F, et al. Future trials of endovascular mechanical recanalisation therapy in acute ischemic stroke patients: a position paper endorsed by ESMINTand ESNR – Part II: methodology of future trials. EJMINT Original Article 2012; http://www.ejmint.org/original-article/1236000072. Published September 4, 2012.3. Baxter B. Review of <strong>Penumbra</strong> System ® Trials: Low rate of embolization of previously uninvolved territory of the brain using the <strong>Penumbra</strong> System in acute ischemic stroke treatment.Poster presented at: 9th Annual Meeting of the Society of NeuroInterventional Surgery; July 23-26, 2012; San Diego, CA, USA.4. Noguiera RG, Lutsep HL, Gupta R, et al.; for the TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke(TREVO2): a randomized trial. Lancet 2012;380:1231-1240.5. Mendes PV, Castaño C, Chapot R, Bonafé A, Andersson T, Gralla J. Solitaire TM FR Revascularization device: A retrospective study as fi rst line device for acute ischemic stroke. Presentedat: 11th Congress of the World Federation of Interventional and Therapeutic Neuroradiology; November 8-11, 2011; Cape Town, South Africa. (Subsequent publication of the study doesnot discuss the data that was presented.)6. Mendes PV, Castaño C, Chapot R, Bonafé A, Andersson T, Gralla J. Solitaire TM FR Revascularization device: A retrospective study as fi rst line device for acute ischemic stroke. Presentedat the 10th Society of NeuroInterventional Surgery Practicum; April 27-28, 2012; New York, NY, USA. (Subsequent publication of the study does not discuss the data that was presented.)www.penumbrainc.com<strong>Penumbra</strong>, <strong>Inc</strong>. USA1351 Harbor Bay PkwyAlameda, CA 94502USA1.888.BRAIN.061.888.272.4606T 1.510.748.3200F 1.510.748.3232order@penumbrainc.cominfo@penumbrainc.com<strong>Penumbra</strong> Europe GmbHAm Borsigturm 4413507 BerlinGermanyT +49 30 2005 676-0F +49 30 2005 676-10order@penumbrainc.deinfo@penumbrainc.de<strong>Penumbra</strong> Neuro Australia PTY LTDSuite 3, Level 5, 1 Oxford StreetDarlinghurst NSW 2010AustraliaT +61-1300 817 025F +61-1300 817 026order.anz@penumbrainc.comCopyright ©2013 <strong>Penumbra</strong>, <strong>Inc</strong>. All rights reserved. The <strong>Penumbra</strong> logo, <strong>Penumbra</strong> System, <strong>Penumbra</strong> System MAX, MAX Tracking Technology, Neuron and Velocity are registered trademarks or trademarks of <strong>Penumbra</strong>, <strong>Inc</strong>. in the USAand other countries. All other brands and product names are registered trademarks or trademarks of their respective owners. PC LI 6127 Rev. B 05/13 USA