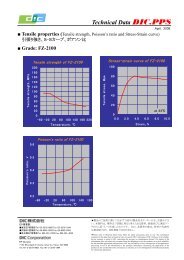
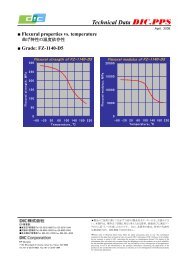
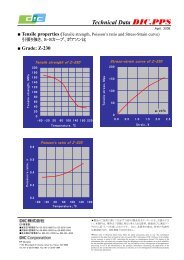
1. é¡æ表é¢ã®åå¦çæ§è³ªã¨åæ£å®å®æ§è©ä¾¡
1. é¡æ表é¢ã®åå¦çæ§è³ªã¨åæ£å®å®æ§è©ä¾¡
1. é¡æ表é¢ã®åå¦çæ§è³ªã¨åæ£å®å®æ§è©ä¾¡
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chemical Properties of Pigment Surface and Dispersion Stability<br />
<br />
Ishimori Motokazu<br />
The pigment-dispersing process consists of three main steps, which are wetting, milling and stabilization<br />
of pigment particles. Stabilization is the most important step among the three steps and<br />
mainly depends on the chemical properties of the pigment surface. In this article, some of the<br />
chemical properties are explained. In addition, the relationship between the stabilization and the<br />
evaluated values or parameters of the pigment surface is also explained.<br />
1 <br />
(1) <br />
[](2) <br />
[](3) [<br />
] <br />
<br />
<br />
<br />
<br />
<br />
(Fig.1)<br />
Fig.1 Image of dispersion and aggregation of particles.<br />
<br />
<br />
<br />
[] <br />
<br />
<br />
<br />
[] <br />
[] <br />
[] <br />
[] <br />
[<br />
] <br />
[] <br />
<br />
<br />
<br />
<br />
DLVO <br />
Vmax/kT>15 <br />
Table 1 <br />
<br />
<br />
<br />
100mV<br />
1) <br />
100mV <br />
<br />
<br />
Table 1 Relation between -potential and Vmax/kT<br />
for Various Particle Radius (r) 1)<br />
- Vmax/kT<br />
potential<br />
(mV) r=1m r=0.1m r=0.01m<br />
25 13 { {<br />
35 26 1 {<br />
50 62 4 {<br />
75 152 11 {<br />
100 286 20 {<br />
150 662 54 4<br />
<br />
<br />
<br />
<br />
<br />
HLB(Hydrophile-Lipophile<br />
Balance) (SP) <br />
DIC Technical Review No.5/1999 1
SP ( d ) ( p )<br />
( h )<br />
2) <br />
3) <br />
<br />
<br />
<br />
<br />
Table 2 <br />
<br />
<br />
Table 2 Relation between Chemical Properties and<br />
Measured Values or Parameters of Pigment Surface<br />
Chemical Property<br />
Measured Value<br />
Parameter Hydrophilic Acidic<br />
/Hydrophobic /Basic<br />
HLB <br />
SP <br />
Amounts of resin adsorption <br />
Titration value of acid or base <br />
Amounts of acid or base adsorption <br />
Isoelectric point <br />
Heat of acid or base adsorption <br />
Ka, Kb <br />
relate partly relate not relate<br />
2 <br />
2.1 HLB<br />
HLB <br />
(1) HLB<br />
20 <br />
HLB = (Wp=Ws) 1 20 (1)<br />
Wp Ws<br />
Ws = 1000Wp = 600 HLB = 12<br />
Pascal 4) HLB <br />
HLB<br />
HLB<br />
(Table 3)<br />
<br />
HLB <br />
<br />
<br />
2.2 (SP)<br />
SP <br />
<br />
<br />
SP <br />
<br />
(1E) <br />
(Vm) <br />
SP <br />
SP Hansen <br />
2;5) SP <br />
( d ) ( p ) ( h ) 3 <br />
70 <br />
(Table 4)<br />
33 <br />
<br />
(Table 5)<br />
53 <br />
SP (Table 6)<br />
<br />
<br />
1 SP <br />
<br />
2 SP SP SP<br />
<br />
SP 42 <br />
<br />
SP <br />
6) SP 31 <br />
<br />
<br />
SP 7) Shareef <br />
SP 8) <br />
15 <br />
3) <br />
SP 3 ( d p h ) <br />
<br />
3 <br />
2.1 HLB <br />
HLB SP <br />
<br />
<br />
SP <br />
<br />
SP SP <br />
<br />
2 DIC Technical Review No.5/1999
Table 3 Required HLB Values - Pigment Colors 4)<br />
Pigments<br />
Required HLB<br />
{Organics{<br />
BON red dark 6 - 8<br />
Toluidine red medium 8 - 10<br />
Toluidine yellow 9 - 11<br />
Phthalocyanine green (yellow shade) 12 - 14<br />
Phthalocyanine green (blue shade) 10 - 12<br />
Phthalocyanine blue (red shade) 11 - 13<br />
Phthalocyanine blue (intermediate shade) 14 - 16<br />
Phthalocyanine blue (intermediate shade) 14 - 16<br />
Phthalocyanine blue (green shade) 14 - 16<br />
\Green-Gold" 11 - 13<br />
Quinacridone violet 11 - 13<br />
Quinacridone red 12 - 14<br />
High strength azo yellow 13 - 15<br />
{Inorganics{<br />
Lampblack 10 - 12<br />
Red iron oxide 13 - 15<br />
Molybdate orange 16 - 18<br />
Rutile titanium dioxide 17 - 20<br />
Chrome yellow medium 18 - 20<br />
Yellow iron oxide 20+<br />
Table 4 Components of the Solubility Parameter<br />
for Solvents 2) Solvents d p h<br />
15 m-Cresol 8.82 3.0 6.1<br />
22A Methylcellosolve 7.9 4.5 7.9<br />
29 Acetone 7.58 5.7 2.0<br />
35 Ethyl acetate 7.44 4.6 2.5<br />
45 Dimethyl formamide 8.52 6.7 5.5<br />
58 Toluene 8.67 <strong>1.</strong>0 2.0<br />
Table 5 Characteristic Parameters for Polymers<br />
and Resins 5)<br />
Polymers o do po ho ao R Ao<br />
B 1<strong>1.</strong>3 9.2 5.0 4.2 6.5 4.0<br />
C 1<strong>1.</strong>5 8.5 5.5 5.5 7.8 4.7<br />
D 9.4 8.5 2.5 3.0 3.9 5.3<br />
E 1<strong>1.</strong>2 9.4 3.2 5.1 6.0 5.0<br />
G 9.8 8.6 3.0 2.0 3.6 3.5<br />
J 10.8 7.0 7.0 4.3 8.2 5.5<br />
[Polymer]<br />
BPoly(methy methacrylate)<br />
CEpikote 1001-epoxy<br />
DPlexal P65-66% oil length alkyd<br />
EPentalyn 830-alcohol soluble rosin resin<br />
GPolystyrene LG<br />
J 1/2 Sec. Nitrocellulose-H 23<br />
SP <br />
9) <br />
<br />
SP<br />
<br />
Table 6 Characteristic Parameters for Various<br />
Pigments 5)<br />
Pigments o do po ho ao R Ao<br />
1 16.8 1<strong>1.</strong>8 7.3 9.5 12.0 8.4<br />
3 10.0 8.7 3.5 3.5 5.0 2.5<br />
7 10.5 9.6 3.0 3.2 4.4 3.9<br />
10 12.0 10.8 3.5 4.0 5.3 5.2<br />
11 12.0 10.0 4.8 4.5 6.6 4.8<br />
14 1<strong>1.</strong>5 9.6 5.2 3.6 6.3 4.4<br />
25 9.1 9.0 2.7 2.3 3.6 2.5<br />
[Pigment]<br />
1TiO2 3C.I.pigment red 48 (Mn)<br />
7C.I.pigment red 57 (Ca) 10C.I.pigment blue 15<br />
11C.I.pigment green 7 14C.I.pigment violet 23<br />
25C.I.pigment yellow 12<br />
<br />
10) <br />
3 <br />
<br />
<br />
Arrhenius <br />
Broensted-Lowry Lewis <br />
<br />
(D N ) <br />
(A N ) 11) Drago <br />
(E A E B ) (C A C B )<br />
12) <br />
DIC Technical Review No.5/1999 3
3.1 <br />
<br />
Sorensen <br />
13) Sorensen Lewis <br />
<br />
<br />
<br />
Lewis <br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
(Table 7)<br />
<br />
<br />
3.2 <br />
<br />
<br />
<br />
<br />
<br />
<br />
3.2.1 <br />
<br />
Fowkes (2) Drago <br />
E A E B C A C B <br />
<br />
12) <br />
PMMA PMMA <br />
<br />
(E B = 3:56C B = 1:99)PMMA <br />
<br />
50 <br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
01H AB E A E B C A C B (2)<br />
3.2.2 <br />
<br />
<br />
<br />
<br />
<br />
14) <br />
<br />
Table 7 Evaluation of Binders 13)<br />
Type Commercial Name Basic Acid Amphoteric Neutral<br />
CELLULOSICS<br />
Cellulose esters CAB *<br />
ACRYLICS<br />
MMA Elvacite 2013 *<br />
EMA Elvacite 2043 *<br />
Polyacrylate Parloid B72 *<br />
Synedol 2263 XB *<br />
POLYAMIDES<br />
Alcohol soluble Versamid 758 ***<br />
POLYURETHANES<br />
Estane 5707 IF *<br />
Polyisocyanate Desmodur N *<br />
Polyisocyanate Desmodur L *<br />
4 DIC Technical Review No.5/1999
15) <br />
3.2.3 <br />
<br />
<br />
28 <br />
n-<br />
<br />
<br />
16) <br />
3.2.4 <br />
<br />
<br />
<br />
<br />
<br />
<br />
17) <br />
<br />
IEP<br />
<br />
<br />
pH <br />
<br />
(Fig.2) 18) <br />
Fig.2 Zeta-potentials of some pigments in various<br />
pH values. 18)<br />
T-1:non treated T-2:acid treated T-4:base treated<br />
pH <br />
<br />
pH <br />
(T-1) <br />
(T-2) <br />
(T-4) <br />
<br />
<br />
TI <br />
<br />
<br />
<br />
<br />
(Fig.3)TI 1 <br />
<br />
IEP TI 1<br />
<br />
IEP <br />
IEP <br />
<br />
Labib (D N ) 9 <br />
11 <br />
D N <br />
(Table 8) 11) <br />
3.2.5 <br />
<br />
<br />
<br />
n-<br />
<br />
<br />
<br />
<br />
<br />
18) <br />
<br />
TI <br />
<br />
<br />
<br />
<br />
3.2.6 (KaKb)<br />
Schreiber Inverse Gas Chromatography(IGC) <br />
(Ka<br />
Kb) <br />
19) <br />
DIC Technical Review No.5/1999 5
Fig.3 Relation of isoelectric points of phthalocyanine pigments and TI values in acidic, basic and amphoteric<br />
resins. 18)<br />
acidic resin basic resin amphoteric resin<br />
<br />
<br />
<br />
(Ka)<br />
(Kb) 20) (3) <br />
(Isp) (0.5
5101) 5g 100ml <br />
pH <br />
10 %<br />
pH DIN/ISO787 <br />
Schroeder <br />
<br />
<br />
<br />
22) <br />
<br />
<br />
<br />
<br />
<br />
() 1999<br />
2 <br />
<br />
<br />
1) : \ 7 ", p. 31, <br />
(1982).<br />
2) C. M. Hansen, J. Paint Technol., 39, (505),104<br />
(1967).<br />
3) , : , 55,459(1982).<br />
4) R. H. Pascal, F. L. Reig, Ocial Digest, 36,839<br />
(1964).<br />
5) C. M. Hansen, J. Paint Technol., 39, (511),505<br />
(1967).<br />
6) , , , , , <br />
: , 47,412(1974).<br />
7) , , : , 67,489(1994).<br />
8) K. M. A. Shareef, M. Yaseen, M. Mahmood. Ali,<br />
P. J. Reddy, J. Coatings Technol., 58, (733),35<br />
(1986).<br />
9) , , : , 62,524(1989).<br />
10) , , : , 63,744(1990).<br />
11) M. E. Labib, R. Williams, J. Colloid Interface Sci.,<br />
97, (2),356(1984).<br />
12) F. M. Fowkes, M. A. Mostafa, Ind. Eng. Chem.<br />
Prod. Res. Dev., 17, (1),3(1978).<br />
13) P. Sorensen, J. Paint Technol., 47(602),31(1975).<br />
14) , , : , 61,692(1988).<br />
15) , : , 67,547(1994).<br />
16) , , : , 52,306(1979).<br />
17) F. M. Fowkes, Dis.Faraday Soc., 42,246(1966).<br />
18) , , : , 65,155(1992).<br />
19) H. P. Schreiber,70th Anniversary Conference on<br />
Colour Materials, Oct.22-24, Tokyo,2C-2V, (1997).<br />
20) U. Panzer, H. P. Schreiber, Macromolecules, 25,<br />
(14),3633(1992).<br />
21) , , : 1994 <br />
, ,10B-07(1994).<br />
22) J. Schroeder, Prog.Org.Coatings, 19,227(1991).<br />
<br />
<br />
<br />
<br />
Ishimori Motokazu<br />
DIC Technical Review No.5/1999 7
8 DIC Technical Review No.5/1999