251 286 - Biotech Bayern
251 286 - Biotech Bayern
251 286 - Biotech Bayern
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
14<br />
Products and Pipeline<br />
Product Pipeline<br />
All the media hype about the failure of GPC <strong>Biotech</strong>´s<br />
product “Satraplatin” with regard to the indication tested<br />
(the treatment of prostate cancer) has not only smashed<br />
this company from the front rank of promising biotech<br />
SMEs, but has also diverted attention from many other<br />
positive developments in Bavarian biotech SMEs.<br />
Bavaria continues to have the only location, Martinsried,<br />
that offers Germany's only approved biotech drugs, i.e.<br />
two drugs manufactured by the company Medigene.<br />
Towards the end of 2007 other Bavarian products had either<br />
already been approved in other countries (IDEA/Switzerland)<br />
or were already at a far advanced phase in the approval<br />
procedure, so that approval can be expected in 2008<br />
(Fresenius/Trion; Medigene).<br />
At present, clinical development work by Bavarian companies<br />
is concerned with the fields of indication listed in<br />
more detail below.<br />
PHASE II<br />
4SC: Rheumatoid Arthritis (RA)<br />
Affectis: Depression/Schizophrenia<br />
Avontec: Asthma, Psoriasis/Dermatitis<br />
Bavarian Nordic: Smallpox, HIV<br />
DoNatur: N.N., N.N.<br />
GPC <strong>Biotech</strong>: NSCLC<br />
IDEA: Onychomycosis<br />
MediGene: Actinic Keratosis,<br />
Pancreas-Ca, Mamma-Ca, RA<br />
Micromet: Mamma-Ca, Acute<br />
Lymphoblastic Leukemia (ALL)<br />
Scil: Implantology<br />
Fresenius <strong>Biotech</strong>/TRION:<br />
Ovarian-Ca, Gastric-Ca, Mamma-Ca<br />
Wilex: Pancreas-Ca, Metastatic<br />
Nephritic-Ca<br />
Antisense Pharma: AP12009,<br />
Glioblastoma<br />
PHASE III<br />
Donatur: N.N., N.N.<br />
Wilex: Nephritic-Ca<br />
(Rencarex®),<br />
PET-Imaging<br />
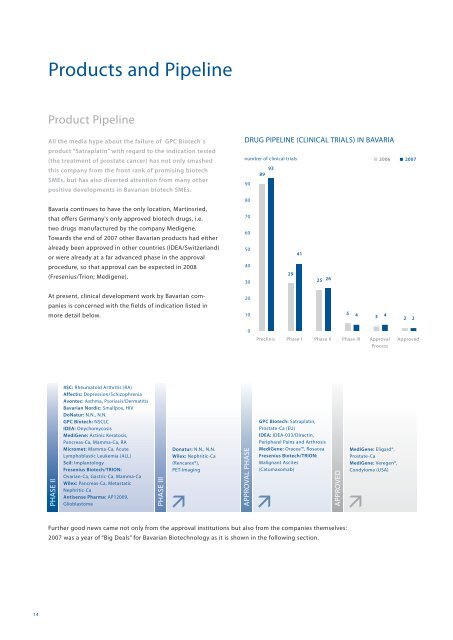
DRUG PIPELINE (CLINICAL TRIALS) IN BAVARIA<br />
number of clinical trials<br />
93<br />
89<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
Preclinic Phase I Phase II Phase III Approval Approved<br />
Process<br />
GPC <strong>Biotech</strong>: Satraplatin,<br />
Prostate-Ca (EU)<br />
IDEA: IDEA-033/Diractin,<br />
Peripharel Pains and Arthrosis<br />
MediGene: Oracea, Rosacea<br />
Fresenius <strong>Biotech</strong>/TRION:<br />
Malignant Ascites<br />
(Catumaxomab)<br />
Further good news came not only from the approval institutions but also from the companies themselves:<br />
2007 was a year of “Big Deals” for Bavarian <strong>Biotech</strong>nology as it is shown in the following section.<br />
APPROVAL PHASE<br />
29<br />
41<br />
25 26<br />
APPROVED<br />
� � �<br />
5 4 3 4<br />
2006 2007<br />
MediGene: Eligard®,<br />
Prostate-Ca<br />
MediGene: Veregen®,<br />
Condyloma (USA)<br />
2 2