Relationship of Glycemia to Cardiovascular Disease ... - Lipids Online
Relationship of Glycemia to Cardiovascular Disease ... - Lipids Online
Relationship of Glycemia to Cardiovascular Disease ... - Lipids Online
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Relationship</strong> <strong>of</strong> <strong>Glycemia</strong> <strong>to</strong><br />
<strong>Cardiovascular</strong> <strong>Disease</strong>: Role <strong>of</strong><br />
Antidiabetic Agents in<br />
<strong>Cardiovascular</strong> Risk Reduction<br />
Richard W. Nes<strong>to</strong>, MD<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
<strong>Relationship</strong> <strong>of</strong> <strong>Glycemia</strong> <strong>to</strong> <strong>Cardiovascular</strong> <strong>Disease</strong>: Role <strong>of</strong> Antidiabetic Agents in<br />
<strong>Cardiovascular</strong> Risk Reduction<br />
Unadjusted Mortality According <strong>to</strong><br />
Glucose Metabolism: Data from AusDiab<br />
Cumulative Incidence <strong>of</strong><br />
All-cause Mortality<br />
0.15<br />
0.10<br />
0.05<br />
All-Cause Mortality<br />
KDM<br />
NDM<br />
IGT<br />
IFG<br />
NGT<br />
0.00<br />
0 2 4 6<br />
Time (years)<br />
Cumulative Incidence <strong>of</strong><br />
CVD Mortality<br />
0.05<br />
0.04<br />
0.03<br />
0.02<br />
0.01<br />
0.00<br />
Reprinted from Barr EL, et al. Circulation. 2007;116:151–157,<br />
with permission from Lippincott Williams & Wilkins.<br />
CVD Mortality<br />
0 2 4 6<br />
Time (years)<br />
AusDiab = Australian Diabetes, Obesity, and Lifestyle Study; CVD = cardiovascular;<br />
KDM = known diabetes mellitus; NDM = newly diagnosed diabetes mellitus; IFG =<br />
impaired fasting glucose; IGT = impaired glucose <strong>to</strong>lerance; NGT = normal glucose<br />
<strong>to</strong>lerance<br />
KDM<br />
NDM<br />
IFG<br />
IGT<br />
NGT<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
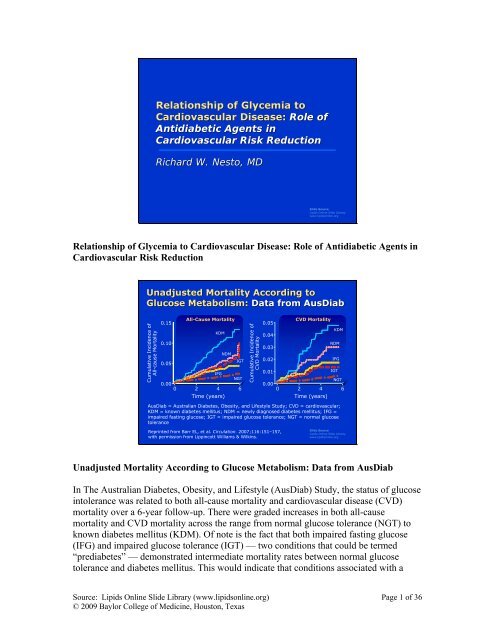
Unadjusted Mortality According <strong>to</strong> Glucose Metabolism: Data from AusDiab<br />
In The Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study, the status <strong>of</strong> glucose<br />
in<strong>to</strong>lerance was related <strong>to</strong> both all-cause mortality and cardiovascular disease (CVD)<br />
mortality over a 6-year follow-up. There were graded increases in both all-cause<br />
mortality and CVD mortality across the range from normal glucose <strong>to</strong>lerance (NGT) <strong>to</strong><br />
known diabetes mellitus (KDM). Of note is the fact that both impaired fasting glucose<br />
(IFG) and impaired glucose <strong>to</strong>lerance (IGT) — two conditions that could be termed<br />
“prediabetes” — demonstrated intermediate mortality rates between normal glucose<br />
<strong>to</strong>lerance and diabetes mellitus. This would indicate that conditions associated with a<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 1 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
high risk for future diabetes could be considered targets for intervention in order <strong>to</strong><br />
reduce mortality. The exact fac<strong>to</strong>rs that govern risk within prediabetes are under<br />
evaluation and are still being debated. These fac<strong>to</strong>rs could include “traditional fac<strong>to</strong>rs,”<br />
such as dyslipidemia, hypertension, dysglycemia, and the proinflamma<strong>to</strong>ry and<br />
prothrombotic fac<strong>to</strong>rs associated with insulin resistance.<br />
Reference:<br />
Barr EL, Zimmet PZ, Welborn TA, et al. Risk <strong>of</strong> cardiovascular and all-cause mortality<br />
in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose<br />
<strong>to</strong>lerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation.<br />
2007;116:151-157.<br />
TNT Study: Substantial Risk <strong>of</strong> <strong>Cardiovascular</strong><br />
Events Persists in Patients With CHD Despite<br />
Treatment With a Maximal Dose Statin<br />
Patients With Major<br />
<strong>Cardiovascular</strong> Event (%)<br />
20<br />
All Metabolic Syndrome<br />
18<br />
A<strong>to</strong>rvastatin 10 mg (n = 2820)<br />
16<br />
A<strong>to</strong>rvastatin 80 mg (n = 2764)<br />
P < 0.0001<br />
14<br />
All Metabolic Syndrome, No Diabetes<br />
12<br />
A<strong>to</strong>rvastatin 10 mg (n = 2191)<br />
10<br />
A<strong>to</strong>rvastatin 80 mg (n = 2162)<br />
8<br />
P = 0.0002<br />
6<br />
4<br />
2<br />
Residual Risk<br />
0<br />
0 1 2 3 4 5 6<br />
Time <strong>to</strong> First Major <strong>Cardiovascular</strong> Event (Years)<br />
CHD = coronary heart disease; TNT = Treating <strong>to</strong> New Targets study<br />
Reprinted from Deedwania P, et al. Lancet 2006;<br />
368:919–928, with permission from Elsevier.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
TNT Study: Substantial Risk <strong>of</strong> <strong>Cardiovascular</strong> Events Persists in Patients With<br />
CHD Despite Treatment With a Maximal Dose Statin<br />
In the subgroup <strong>of</strong> patients with metabolic syndrome and diabetes in the Treating <strong>to</strong> New<br />
Targets study, 80 mg <strong>of</strong> a<strong>to</strong>rvastatin was more effective than 10 mg <strong>of</strong> a<strong>to</strong>rvastatin at<br />
reducing the risk <strong>of</strong> major cardiovascular events. Although there was a robust reduction<br />
in risk among the patients who received the maximal dose <strong>of</strong> a<strong>to</strong>rvastatin, a substantial<br />
residual risk remained despite maximal lowering <strong>of</strong> low-density lipoprotein. Furthermore,<br />
the residual risk <strong>of</strong> patients with metabolic syndrome and diabetes was nearly double that<br />
in patients without these metabolic disturbances.<br />
Reference:<br />
Deedwania P, Barter P, Carmena R, et al, for the Treating <strong>to</strong> New Targets Investiga<strong>to</strong>rs.<br />
Reduction <strong>of</strong> low-density lipoprotein cholesterol in patients with coronary heart disease<br />
and metabolic syndrome: analysis <strong>of</strong> the Treating <strong>to</strong> New Targets study. Lancet.<br />
2006;368:919-928.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 2 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
TNT Study: Impact <strong>of</strong> Glucometabolic<br />
Characteristics on Risk <strong>of</strong> Major<br />
<strong>Cardiovascular</strong> Events Among All Patients<br />
Patients with Major<br />
<strong>Cardiovascular</strong> Event (%)<br />
16<br />
12<br />
8<br />
4<br />
0<br />
Low<br />
High-Density<br />
Lipoprotein<br />
Characteristic Absent<br />
Characteristic Present<br />
HR = 1.33<br />
HR = 1.30<br />
Fasting<br />
Glucose<br />
100 mg/dL<br />
HR = 1.24 HR = 1.18 HR = 1.48<br />
Body-Mass<br />
Index<br />
28 kg/m 2<br />
Triglycerides<br />
250 mg/dL<br />
Hypertension<br />
TNT = Treating <strong>to</strong> New Targets study<br />
Reprinted from Deedwania P, et al. Lancet. 2006;<br />
368:919–928, with permission from Elsevier.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
TNT Study: Impact <strong>of</strong> Glucometabolic Characteristics on Risk <strong>of</strong> Major<br />
<strong>Cardiovascular</strong> Events Among All Patients<br />
The fac<strong>to</strong>rs associated with residual risk are the criteria commonly used <strong>to</strong> make a<br />
diagnosis <strong>of</strong> metabolic syndrome. According <strong>to</strong> the Treating <strong>to</strong> New Targets study,<br />
patients who have low levels <strong>of</strong> high-density lipoprotein, a fasting glucose >100 mg/dL,<br />
obesity, hypertriglyceridemia, and hypertension are placed at risk despite the lowering <strong>of</strong><br />
low-density lipoprotein with high-dose a<strong>to</strong>rvastatin. These 5 fac<strong>to</strong>rs could then represent<br />
additional targets for intervention with the hope that some <strong>of</strong> the residual risk could be<br />
reduced. At present, attention has been directed <strong>to</strong> the possibility that an intervention that<br />
lowers fasting glucose could remove some <strong>of</strong> this residual risk.<br />
Reference:<br />
Deedwania P, Barter P, Carmena R, et al, for the Treating <strong>to</strong> New Targets Investiga<strong>to</strong>rs.<br />
Reduction <strong>of</strong> low-density lipoprotein cholesterol in patients with coronary heart disease<br />
and metabolic syndrome: analysis <strong>of</strong> the Treating <strong>to</strong> New Targets study. Lancet.<br />
2006;368:919-928.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 3 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
UKPDS: Hemoglobin A 1c and Rates for Myocardial<br />
Infarction and Microvascular Complications<br />
Adjusted Incidence per<br />
1,000 Person Years (%)<br />
80<br />
60<br />
40<br />
20<br />
Myocardial infarction<br />
Microvascular endpoints<br />
0<br />
5 6 7 8 9 10 11<br />
Updated Mean HbA 1c Concentration<br />
HbA 1c = hemoglobin A 1c (glycosylated)<br />
UKPDS = United Kingdom Prospective Diabetes Study<br />
Strat<strong>to</strong>n IM, et al. BMJ. 2000;321:405–412; reproduced<br />
with permission from the BMJ Publishing Group.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
UKPDS: Hemoglobin A 1c and Rates for Myocardial Infarction and Microvascular<br />
Complications<br />
In United Kingdom Prospective Diabetes Study, which examined the updated mean<br />
hemoglobin A 1c (HbA 1c ) on treatment and the incidence <strong>of</strong> both myocardial infarction<br />
(MI) and microvascular endpoints, there were graded increases across glycemia for the<br />
risk <strong>of</strong> these end-organ complications. The risk <strong>of</strong> microvascular disease was much<br />
greater than the risk for MI at the same HbA 1c concentration. These data support<br />
observations, over the years, that reductions in HbA 1c with treatment could produce<br />
robust decreases in microvascular endpoints. The slope <strong>of</strong> the curve <strong>of</strong> glycemic control<br />
related <strong>to</strong> MI risk is not as steep, indicating that if intervention is <strong>to</strong> be successful in<br />
reducing MI risk, then larger decreases in HbA 1c may be required. Of note is the fact that<br />
across the range <strong>of</strong> HbA 1c concentrations from 5.5% <strong>to</strong> 9.5%, the absolute risk for MI<br />
was greater than the risk for the development <strong>of</strong> microvascular disease. The mechanism<br />
by which glycemia increases risk for vascular disease has been the subject <strong>of</strong> intense<br />
scrutiny.<br />
Reference:<br />
Strat<strong>to</strong>n IM, Adler AI, Neil HA, et al. Association <strong>of</strong> glycaemia with macrovascular and<br />
microvascular complications <strong>of</strong> type 2 diabetes (UKPDS 35): prospective observational<br />
study. BMJ. 2000;321:405-412.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 4 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
The Proinflamma<strong>to</strong>ry Milieu in Atheromata<br />
Is Mediated by RAGEs in Diabetes Mellitus<br />
RAGE = recep<strong>to</strong>r for advanced glycosylated end-products<br />
Reprinted from Cipollone F, et al. Circulation. 2003;108:1070–1077,<br />
with permission from Lippincott Williams & Wilkins.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
The Proinflamma<strong>to</strong>ry Milieu in Atheromata Is Mediated by RAGEs in Diabetes<br />
Mellitus<br />
In this study by Cipollone et al., carotid endarterec<strong>to</strong>my specimens from diabetic patients<br />
were found <strong>to</strong> be distinctly different from those <strong>of</strong> nondiabetic patients. This study also<br />
provided in-vivo data demonstrating that atheromata in diabetic patients may have a<br />
different pathobiology. The diabetic carotid atheroma specimens contained localized<br />
dense infiltrates <strong>of</strong> mononuclear cells expressing surface markers consistent with active<br />
inflammation. The specimens from the nondiabetic patients did not show this degree <strong>of</strong><br />
inflamma<strong>to</strong>ry infiltrate. Monocytes, macrophages, and lymphocytes reside in this region<br />
<strong>of</strong> the plaque because the recep<strong>to</strong>rs for advanced glycosylated end-products (RAGEs) are<br />
located in these areas, and the attachment <strong>of</strong> the RAGEs <strong>to</strong> the recep<strong>to</strong>r induces a<br />
chemotactic response accounting for the inflamma<strong>to</strong>ry appearance <strong>of</strong> atheroma<br />
specimens from diabetic patients. Furthermore, the density <strong>of</strong> the RAGEs was related <strong>to</strong><br />
the level <strong>of</strong> glycemia in these particular patients, thus providing a link between glycemia<br />
and vascular inflammation. The specimens from the diabetic patients expressed high<br />
concentrations <strong>of</strong> matrix metallinoproteinases in the area <strong>of</strong> inflamma<strong>to</strong>ry cells, but those<br />
<strong>of</strong> the nondiabetic patients did not.<br />
Let us now examine the possibility that the pharmacologic strategies used <strong>to</strong> lower<br />
glucose in both prediabetes and diabetes can reduce the risk <strong>of</strong> cardiovascular disease.<br />
Reference:<br />
Cipollone F, Iezzi A, Fazia M, et al. The recep<strong>to</strong>r RAGE as a progression fac<strong>to</strong>r<br />
amplifying arachidonate-dependent inflamma<strong>to</strong>ry and proteolytic response in human<br />
atherosclerotic plaques: role <strong>of</strong> glycemic control. Circulation. 2003;108:1070-1077.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 5 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Effect <strong>of</strong> Acarbose on the Probability <strong>of</strong><br />
Remaining Free <strong>of</strong> <strong>Cardiovascular</strong> <strong>Disease</strong><br />
Probability <strong>of</strong> Any<br />
<strong>Cardiovascular</strong> Event<br />
0.06<br />
0.05<br />
0.04<br />
0.03<br />
0.02<br />
0.01<br />
P = 0.04 (Log-Rank Test)<br />
P = 0.03 (Cox Proportional Model)<br />
Placebo<br />
Acarbose<br />
0.00<br />
0<br />
No. at Risk<br />
Placebo 686<br />
Acarbose 682<br />
100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400<br />
675<br />
659<br />
667<br />
635<br />
658<br />
622<br />
643<br />
608<br />
Days After Randomization<br />
638<br />
601<br />
633<br />
596<br />
627<br />
590<br />
615<br />
577<br />
611<br />
567<br />
604<br />
558<br />
519<br />
473<br />
424<br />
376<br />
332 232<br />
286 203<br />
Chiasson JL, et al. JAMA. 2003;290;486–494. Copyright<br />
© 2003 American Medical Association. All rights reserved.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Effect <strong>of</strong> Acarbose on the Probability <strong>of</strong> Remaining Free <strong>of</strong> <strong>Cardiovascular</strong> <strong>Disease</strong><br />
In the STOP-NIDDIM trial, Chiasson et al. (2002) showed that patients with an impaired<br />
glucose <strong>to</strong>lerance who were randomized <strong>to</strong> receive acarbose had a lower rate <strong>of</strong><br />
conversion <strong>to</strong> type 2 diabetes over 3 years. In addition, the authors examined the effect <strong>of</strong><br />
acarbose on the development <strong>of</strong> major cardiovascular events, including coronary heart<br />
disease (myocardial infarction, new angina, revascularization procedures), cardiovascular<br />
death, congestive heart failure, cerebrovascular events, and peripheral vascular disease.<br />
These subjects also showed, with a significant P value, a lower risk for any<br />
cardiovascular event.<br />
References:<br />
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, for the STOP-<br />
NIDDM Trial Research Group. Acarbose treatment and the risk <strong>of</strong> cardiovascular disease<br />
and hypertension in patients with impaired glucose <strong>to</strong>lerance: the STOP-NIDDM trial.<br />
JAMA. 2003;290:486-494.<br />
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, for the STOP-<br />
NIDDM Trial Research Group. Acarbose for prevention <strong>of</strong> type 2 diabetes mellitus: the<br />
STOP-NIDDM randomised trial. Lancet. 2002;359:2072-2077.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 6 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
STOP-NIDDM Trial: Acarbose Prevents Type 2<br />
Diabetes Mellitus and <strong>Cardiovascular</strong> Events in<br />
Subjects With Impaired Glucose Tolerance<br />
Study Population<br />
Acarbose<br />
n=662<br />
Placebo<br />
n=888<br />
Hazard Ratio<br />
(95% CI)<br />
P<br />
Value<br />
Favors<br />
Acarbose<br />
Favors<br />
Placebo<br />
Myocardial infarction<br />
1<br />
12<br />
0.09 (0.01–0.72)<br />
0.02<br />
Angina<br />
5<br />
12<br />
0.45 (0.16–1.28)<br />
0.13<br />
Revascularization<br />
procedures<br />
11<br />
20<br />
0.61 (0.29–1.26)<br />
0.18<br />
<strong>Cardiovascular</strong> death<br />
1<br />
2<br />
0.55 (0.05–6.11)<br />
0.63<br />
Congestive heart failure<br />
0<br />
2<br />
Cerebrovascular stroke<br />
2<br />
4<br />
0.56 (0.10–3.07)<br />
0.51<br />
Any cardiovascular<br />
event<br />
15<br />
32<br />
0.51 (0.28–0.95)<br />
0.03<br />
CI = confidence interval<br />
0 0.5 1.0 1.5<br />
Hazard Ratio<br />
Chiasson JL, et al. JAMA. 2003;290;486–494. Copyright © 2003<br />
American Medical Association. All rights reserved.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
STOP-NIDDM Trial: Acarbose Prevents Type 2 Diabetes Mellitus and<br />
<strong>Cardiovascular</strong> Events in Subjects With Impaired Glucose Tolerance<br />
Examining the different events between the acarbose and placebo populations in the<br />
STOP-NIDDM trial showed that there were 15 events in the acarbose group and 32<br />
events in the placebo groups, suggesting that a treatment focused on postprandial<br />
hyperglycemia could reduce the risk for cardiovascular events. These intriguing data<br />
await further confirmation and additional studies.<br />
Reference:<br />
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, for the STOP-<br />
NIDDM Trial Research Group. Acarbose treatment and the risk <strong>of</strong> cardiovascular disease<br />
and hypertension in patients with impaired glucose <strong>to</strong>lerance: the STOP-NIDDM trial.<br />
JAMA. 2003;290:486-494.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 7 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Controlling Postprandial Hyperglycemia Leads <strong>to</strong> Regression <strong>of</strong><br />
Carotid Atherosclerosis in Patients With Type 2 Diabetes Mellitus<br />
Repaglinide Glyburide<br />
1.4<br />
*P = 0.01<br />
Postprandial Postprandial 1.3<br />
260<br />
Peak<br />
Peak<br />
1.2<br />
P 0.001 P 0.01<br />
220<br />
1.1<br />
*<br />
180<br />
1.0<br />
*<br />
140<br />
0.9<br />
100<br />
Before After Before After<br />
0.8<br />
260<br />
0.7<br />
220<br />
0.6<br />
180<br />
0.5<br />
140<br />
0.4<br />
100<br />
P 0.001 P 0.01<br />
0<br />
Before After Before After<br />
0 60 120 0 60 120<br />
Minutes<br />
Repaglinide Glyburide<br />
Glucose (mg/dL)<br />
Carotid Intima-Media Thickness<br />
(mm)<br />
C-IMT Regression Associated With PPG, IL-6, and CRP<br />
C-IMT = carotid intima-media thickness; PPG = postprandial glucose; IL-6 = interleukin 6;<br />
CRP = C-reactive protein<br />
Reprinted from Esposi<strong>to</strong> K, et al. Circulation. 2004;110:214–219,<br />
with permission from Lippincott Williams & Wilkins.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Controlling Postprandial Hyperglycemia Leads <strong>to</strong> Regression <strong>of</strong> Atherosclerosis in<br />
Patients With Type 2 Diabetes Mellitus<br />
Esposi<strong>to</strong> et al. (2004) investigated repaglinide, a prandial secretagogue, as a strategy for<br />
reducing postprandial hyperglycemia in patients with type 2 diabetes mellitus. Patients<br />
enrolled in the study were randomized <strong>to</strong> receive either repaglinide or glyburide. Those<br />
who received repaglinide had a reduction in the progression <strong>of</strong> carotid intima-media<br />
thickness when compared <strong>to</strong> those who received glyburide. The data from this study<br />
would support the findings <strong>of</strong> the STOP-NIDDM trial (previous slide).<br />
Reference:<br />
Esposi<strong>to</strong> K, Giugliano D, Nappo F, Marfella R, for the Campanian Postprandial<br />
Hyperglycemia Study Group. Regression <strong>of</strong> carotid atherosclerosis by control <strong>of</strong><br />
postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214-219.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 8 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Controlling Postprandial Hyperglycemia Leads <strong>to</strong><br />
Regression <strong>of</strong> Atherosclerosis in Patients With<br />
Type 2 Diabetes Mellitus (Continued)<br />
• 78 drug-naïve type 2 diabetes mellitus patients with hemoglobin A 1c
Glycemic Control and Macrovascular<br />
<strong>Disease</strong> in Patients With Type 1 or Type 2<br />
Diabetes: Meta-analysis analysis <strong>of</strong> Clinical Trials<br />
Trials<br />
Holman<br />
Verrillo<br />
Lauritzen<br />
Feldt-<br />
Rasmussen<br />
DCCT PP<br />
DCCT SI<br />
SDIS<br />
MCSG<br />
Overall<br />
Trials in Type 1 Diabetes<br />
Favors<br />
Intensified<br />
Glycemic<br />
Control<br />
Favors<br />
Conventional<br />
Glycemic<br />
Control<br />
0.01 0.1 .5 1 2 10 100<br />
Incidence Rate Ratio<br />
%<br />
Weight<br />
1.5<br />
6.6<br />
1.5<br />
1.7<br />
35.4<br />
44.0<br />
7.8<br />
1.5<br />
100.0<br />
Reprinted from Stettler C, et al. Am Heart J. 2006;<br />
152:27–38, with permission from Elsevier.<br />
Trials in Type 2 Diabetes<br />
Favors Favors<br />
Intensified Conventional<br />
Glycemic Glycemic<br />
Trials<br />
Control Control<br />
Veterans<br />
Affairs<br />
UKPDS 1<br />
UKPDS 2<br />
UKPDS 3<br />
Kumamo<strong>to</strong> PP<br />
Kumamo<strong>to</strong> SI<br />
Overall<br />
0.01 0.1 0.5 1 2 10 100<br />
Incidence Rate Ratio<br />
%<br />
Weight<br />
4.3<br />
53.6<br />
27.2<br />
14.3<br />
0.1<br />
0.5<br />
100.0<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Glycemic Control and Macrovascular <strong>Disease</strong> in Patients With Type 1 or Type 2<br />
Diabetes: Meta-analysis <strong>of</strong> Clinical Trials<br />
Stettler et al. (2006) evaluated those trials in which pharmacologic treatment <strong>of</strong><br />
hyperglycemia in patients with type 2 diabetes was evaluated with respect <strong>to</strong><br />
macrovascular disease. They found that, at best, intensified glycemic control was<br />
associated with only a modest reduction in macrovascular disease. Although these data<br />
are promising, none <strong>of</strong> the trials that were evaluated showed the degree <strong>of</strong> dramatic<br />
reduction that one might expect based on the epidemiological correlation between the<br />
level <strong>of</strong> glycemia and cardiovascular risk.<br />
Reference:<br />
Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types<br />
1 and 2 diabetes mellitus: meta-analysis <strong>of</strong> randomized trials. Am Heart J. 2006;152:27-<br />
38.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 10 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
DCCT-EDIC:<br />
Long-term Risk <strong>of</strong><br />
Macrovascular Complications<br />
Hemoglobin A 1C<br />
12%<br />
10%<br />
8%<br />
6%<br />
Conventional<br />
Intensive<br />
P < 0.001 P < 0.001 P = 0.61<br />
Cumulative Incidence<br />
0.12<br />
0.10<br />
0.08<br />
0.06<br />
0.04<br />
0.02<br />
Any <strong>Cardiovascular</strong> Outcome<br />
42% risk reduction<br />
P = 0.02<br />
Conventional<br />
Intensive<br />
DCCT<br />
End <strong>of</strong><br />
Randomized<br />
Treatment<br />
EDIC<br />
Year 1<br />
EDIC<br />
Year 7<br />
0.00<br />
0 2 4 6 8 10 12 14 16 18 20<br />
Years Since Entry*<br />
*Diabetes Control and Complications Trial (DCCT) ended and Epidemiology <strong>of</strong> Diabetes<br />
Interventions and Complications (EDIC) began in year 10 (1993). Mean follow-up: 17 years.<br />
DCCT/EDIC Research Group. JAMA. 2002;287:2563-2569. Copyright © 2002<br />
American Medical Association. All rights reserved. | Nathan DM, et al. N Engl J<br />
Med. 2005;353:2643-2653. Copyright © 2005 Massachusetts Medical Society.<br />
All rights reserved.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
DCCT-EDIC: Long-term Risk <strong>of</strong> Macrovascular Complications<br />
At the end <strong>of</strong> the randomized treatment phase in the Diabetes Control and Complications<br />
Trial, the research group found a difference in the concentration <strong>of</strong> hemoglobin A 1c<br />
between the patients with type 1 diabetes in the intensive treatment group and those in the<br />
conventional treatment group. At the end <strong>of</strong> the trial, there was a nonsignificant reduction<br />
in cardiovascular outcome in the intensively treated group. The trial ended at<br />
approximately 9 years; afterward, there was convergence <strong>of</strong> treatments and similar levels<br />
<strong>of</strong> glycemic control were achieved. There was persistent benefit, however, among the<br />
intensively treated group such that there was a statistically significant reduction in<br />
cardiovascular disease when compared <strong>to</strong> the conventionally treated group in the followup<br />
phase (up <strong>to</strong> 20 years) <strong>of</strong> the study. These data would indicate that 10 years <strong>of</strong><br />
intensive treatment yielded a cardiovascular benefit during the first 10 years that was<br />
sustained and became greater in the follow-up phase.<br />
References:<br />
Diabetes Control and Complications Trial/Epidemiology <strong>of</strong> Diabetes Interventions and<br />
Complications Research Group. Effect <strong>of</strong> intensive therapy on the microvascular<br />
complications <strong>of</strong> type 1 diabetes mellitus. JAMA. 2002;287:2563-2569.<br />
Nathan DM, Cleary PA, Backlund JY, et al, for the Diabetes Control and Complications<br />
Trial/Epidemiology <strong>of</strong> Diabetes Interventions and Complications (DCCT/EDIC) Study<br />
Research Group. Intensive diabetes treatment and cardiovascular disease in patients with<br />
type 1 diabetes. N Engl J Med. 2005;353:2643-2653.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 11 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
UKPDS: Metformin Is Associated With a<br />
Reduction in <strong>Cardiovascular</strong> Events<br />
9<br />
Glibenclamide Insulin<br />
Metformin<br />
Sulfonylurea/<br />
Insulin<br />
Hemoglobin A 1c<br />
8<br />
7<br />
6<br />
Conventional<br />
Chlorpropamide<br />
Metformin<br />
5<br />
0 2 4 6 8 10<br />
Years<br />
UKPDS = United Kingdom Prospective<br />
Diabetes Study<br />
Reprinted in an adapted form from UKPDS Group. Lancet<br />
1998;352:854–865, with permission from Elsevier.<br />
Diabetes-related<br />
death<br />
All-cause<br />
mortality<br />
Any diabetesrelated<br />
endpoint<br />
Myocardial<br />
infarction<br />
Stroke<br />
Mean<br />
Change<br />
in Risk*<br />
42%<br />
36%<br />
32%<br />
39%<br />
41%<br />
P Value<br />
0.017<br />
0.011<br />
0.002<br />
0.010<br />
0.13<br />
Mean<br />
Change<br />
in Risk*<br />
20%<br />
8%<br />
7%<br />
21%<br />
14%<br />
P Value<br />
0.19<br />
0.49<br />
0.46<br />
0.11<br />
0.6<br />
*Compared with conventional therapy based on<br />
diet/exercise in overweight patients<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
UKPDS: Metformin Is Associated With a Reduction in <strong>Cardiovascular</strong> Events<br />
In the United Kingdom Prospective Diabetes Study, metformin emerged as one treatment<br />
for diabetes that was associated with a decrease in both myocardial infarction and stroke.<br />
Despite the similar concentrations <strong>of</strong> hemoglobin A 1c achieved with insulin, various<br />
sulfonylureas and metformin, the use <strong>of</strong> metformin in the small subgroup <strong>of</strong> patients with<br />
obesity did show this cardiovascular benefit. These data helped <strong>to</strong> solidify the belief that<br />
the use <strong>of</strong> metformin as an initial therapy in the treatment <strong>of</strong> type 2 diabetes may be<br />
preferable from a cardiovascular point <strong>of</strong> view.<br />
Reference:<br />
UK Prospective Diabetes Study (UKPDS) Group. Effect <strong>of</strong> intensive blood-glucose<br />
control with metformin on complications in overweight patients with type 2 diabetes<br />
(UKPDS 34). Lancet. 1998;352:854-865.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 12 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Non–UKPDS Trials:<br />
Metformin vs. Other Interventions<br />
RR (Fixed) 95% CI<br />
Study<br />
All-cause mortality<br />
DeFronzo 1995<br />
Hor<strong>to</strong>n 2000<br />
Sub<strong>to</strong>tal (95% CI)<br />
MET<br />
n/N<br />
1/210<br />
1/178<br />
388<br />
Favours<br />
Comparison Metformin<br />
0/209<br />
0/172<br />
381<br />
Favours Weight<br />
Comparison (%)<br />
49.6<br />
50.4<br />
100.0<br />
RR (fixed)<br />
95% CI<br />
2.99 [0.12, 72.88]<br />
2.90 [0.12, 70.69]<br />
2.94 [0.31, 28.16]<br />
Ischemic heart disease<br />
DeFronzo 1995<br />
1/210<br />
0/209<br />
25.2<br />
2.99 [0.12, 72.88]<br />
Hallsten 2002<br />
1/13<br />
0/14<br />
24.2<br />
3.21 [0.14, 72.55]<br />
Hor<strong>to</strong>n 2000<br />
1/178<br />
0/172<br />
25.5<br />
2.90 [0.12, 70.69]<br />
Teupe 1991<br />
1/50<br />
0/50<br />
25.1<br />
3.00 [0.13, 71.92]<br />
Sub<strong>to</strong>tal (95% CI)<br />
451<br />
445<br />
100.0<br />
3.02 [0.62, 14.75]<br />
0.01 0.1 1 10 100<br />
CI = confidence interval; MET = metformin; RR = relative risk;<br />
UKPDS = United Kingdom Prospective Diabetes Study<br />
Reprinted from Saenz A, et al. Cochrane Database Syst Rev.<br />
2005;(3):CD002966, with permission from Wiley.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Non–UKPDS Trials: Metformin vs. Other Interventions<br />
When one looks at metformin data in studies outside <strong>of</strong> the United Kingdom Prospective<br />
Diabetes Study, however, there apparently is no evidence <strong>of</strong> cardiovascular benefit, as in<br />
this meta-analysis conducted by Saenz et al. (2005). Furthermore, these other studies tend<br />
<strong>to</strong> have small numbers <strong>of</strong> patients.<br />
Reference:<br />
Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin<br />
monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev.<br />
2005;(3):CD002966.<br />
Non–UKPDS Trials:<br />
Metformin vs. Conventional Treatment<br />
RR (Fixed) 95% CI<br />
UKPDS<br />
Any diabetes-related<br />
outcomes<br />
MET<br />
n/N<br />
98/342<br />
Conventional Favours<br />
n/N metformin<br />
160/411<br />
Favours Weight<br />
comparison (%)<br />
100.0<br />
RR (fixed)<br />
95% CI<br />
0.74 [0.60, 0.90]<br />
Diabetes-related<br />
death<br />
28/342<br />
55/411<br />
100.0<br />
0.61 [0.40, 0.94]<br />
All-cause mortality<br />
50/342<br />
89/411<br />
100.0<br />
0.68 [0.49, 0.93]<br />
Myocardial infarction<br />
39/342<br />
73/411<br />
100.0<br />
0.64 [0.45, 0.92]<br />
Stroke<br />
12/342<br />
23/411<br />
100.0<br />
0.63 [0.32, 1.24]<br />
Peripheral vascular<br />
disease<br />
6/342<br />
9/411<br />
100.0<br />
0.80 [0.29, 2.23]<br />
Microvascular<br />
24/342<br />
38/411<br />
100.0<br />
0.76 [0.46, 1.24]<br />
0.2 0.5 1 2 5<br />
CI = confidence interval; MET = metformin; RR = relative risk;<br />
UKPDS = United Kingdom Prospective Diabetes Study<br />
Reprinted from Saenz A, et al. Cochrane Database Syst Rev.<br />
2005;(3):CD002966, with permission from Wiley.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 13 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Non-UKPDS Trials: Metformin vs. Conventional Treatment<br />
When one looks at metformin data in studies outside <strong>of</strong> the United Kingdom Prospective<br />
Diabetes Study, however, there apparently is no evidence <strong>of</strong> cardiovascular benefit, as in<br />
this meta-analysis conducted by Saenz et al. (2005). Furthermore, these other studies tend<br />
<strong>to</strong> have small numbers <strong>of</strong> patients.<br />
Reference:<br />
Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin<br />
monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev.<br />
2005;(3):CD002966.<br />
Nissen and Wolski Meta-analysis:<br />
analysis:<br />
Rates <strong>of</strong> Myocardial Infarction and<br />
Death from <strong>Cardiovascular</strong> Causes<br />
UKPDS<br />
Rosiglitazone<br />
Control<br />
Odds Ratio<br />
(95% CI)<br />
p<br />
value<br />
Myocardial Infarction<br />
Small trials<br />
44/10,285 (0.43%)<br />
22/6105 (0.36%)<br />
1.45 (0.88–2.39)<br />
0.15<br />
DREAM<br />
15/2,635 (0.57%)<br />
9/2634 (0.34%)<br />
1.65 (0.74–3.68)<br />
0.22<br />
ADOPT<br />
27/1,456 (1.85%)<br />
41/2895 (1.44%)<br />
1.33 (0.80–2.21)<br />
0.27<br />
Total<br />
86/14371 (0.60%)<br />
72/11634 (0.62%) 1.43 (1.03–1.98)<br />
0.03<br />
Death from <strong>Cardiovascular</strong> Causes<br />
Small trials<br />
25/6,845 (0.36%)<br />
7/3,980 (0.18%)<br />
2.40 (1.17–4.91)<br />
0.02<br />
DREAM<br />
12/2,635 (0.46%)<br />
10/2,634 (0.38%)<br />
1.20 (0.52–2.78)<br />
0.67<br />
ADOPT<br />
2/1,456 (0.14%)<br />
5/2,895 (0.17%)<br />
0.80 (0.17–3.86)<br />
0.78<br />
Total<br />
39/10648 (0.37%)<br />
22/9188 (0.24%)<br />
1.64 (0.98–2.74)<br />
0.06<br />
CI = confidence interval; UKPDS = United Kingdom Prospective Diabetes Study<br />
Nissen SE, Wolski K. N Engl J Med. 2007; 356. 2457–2471. Copyright<br />
© 2007 Massachusetts Medical Society. All rights reserved.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Nissen and Wolski Meta-analysis: Rates <strong>of</strong> Myocardial Infarction and Death from<br />
<strong>Cardiovascular</strong> Causes<br />
Based on their meta-analysis <strong>of</strong> the cardiovascular safety <strong>of</strong> rosiglitazone, Nissen and<br />
Wolski (2007) raised the question <strong>of</strong> whether there is a risk for myocardial infarction<br />
among diabetic patients treated with rosiglitazone versus other treatments. In the studies<br />
they evaluated, cardiovascular endpoints were neither adjudicated nor were they primary<br />
endpoints; the mean follow-up period for most <strong>of</strong> the evaluated studies was only 6<br />
months. These observations raised concern about whether the preclinical studies <strong>of</strong><br />
rosiglitazone, which had shown the drug <strong>to</strong> have antiatherosclerotic affects, were relevant<br />
<strong>to</strong> patients with type 2 diabetes. Since then, there have been numerous studies whose<br />
findings conflict with those <strong>of</strong> this meta-analysis.<br />
Reference:<br />
Nissen SE, Wolski K. Effect <strong>of</strong> rosiglitazone on the risk <strong>of</strong> myocardial infarction and<br />
death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 14 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Balance <strong>of</strong> Evidence: Is Rosiglitazone<br />
Associated With <strong>Cardiovascular</strong> Risk?<br />
MI = myocardial infarction<br />
MET = metformin<br />
SU = sulfonylurea<br />
PIO = pioglitazone<br />
FDA Analysis<br />
MI signal with<br />
insulin and nitrates<br />
GSK ICT analysis<br />
MI signal<br />
Nissen et al.<br />
MI signal<br />
ACCORD<br />
Mortality not linked <strong>to</strong> <strong>to</strong> any<br />
specific diabetes drug<br />
ADOPT<br />
No significant risk<br />
Comparable <strong>to</strong> <strong>to</strong> MET, SU<br />
Lago et et al. Lancet.<br />
No significant risk<br />
Comparable <strong>to</strong> <strong>to</strong> PIO<br />
RECORD<br />
No significant risk<br />
Comparable <strong>to</strong> <strong>to</strong> MET, SU<br />
McAfee et et al.<br />
No significant risk<br />
Comparable <strong>to</strong> <strong>to</strong> MET/SU<br />
Rosen et al.<br />
No significant risk<br />
Comparable <strong>to</strong> <strong>to</strong> PIO<br />
“…[W]e believe that only prospective clinical trials designed for the specific<br />
purpose <strong>of</strong> establishing the cardiovascular benefit or risk <strong>of</strong> rosiglitazone<br />
will resolve the controversy about its safety.” — Diamond et al., 2007<br />
Diamond GA, et al. Ann Intern Med. 2007;147:578–581 | Home PD. N Engl J Med.<br />
2007;357:28–38 | Krall RL. Lancet. 2007;369:1995–1996. | Lago RM, et al. Lancet.<br />
2007;370:1129–1136 | McAfee AT, et al. Pharmacoepidemiol Drug Saf. 2007;<br />
16:711–725 | Nissen SE, et al. N Engl J Med. 2007;356:2457–2471 | Rosen CJ.<br />
N Engl J Med. 2007;357:844–846.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Balance <strong>of</strong> Evidence: Is Rosiglitazone Associated With <strong>Cardiovascular</strong> Risk?<br />
Recently published data from the ACCORD and ADVANCE studies — both <strong>of</strong> which<br />
evaluated whether lowering the hemoglobin A 1c concentration (below its current<br />
recommended target <strong>of</strong> 7%) is associated with cardiovascular benefit — did not show any<br />
link between rosiglitazone and an excess risk for myocardial infarction. Furthermore, a<br />
meta-analysis conducted by Lago et al. (2007) did not show any significant risk for<br />
cardiovascular death when they compared rosiglitazone <strong>to</strong> other treatments, particularly<br />
pioglitazone. The interim analysis <strong>of</strong> the RECORD trial also showed that rosiglitazone<br />
carries a risk for cardiovascular disease that is comparable <strong>to</strong> metformin and<br />
sulfonylureas.<br />
ACCORD = Action <strong>to</strong> Control <strong>Cardiovascular</strong> Risk in Diabetes<br />
ADVANCE = Action in Diabetes and Vascular <strong>Disease</strong>: Preterax and Diamicron MR<br />
Controlled Evaluation<br />
RECORD = Rosiglitazone Evaluated for Cardiac Outcomes and Regulation <strong>of</strong> <strong>Glycemia</strong><br />
in Diabetes<br />
References:<br />
Diamond GA, Bax L, Kaul S. Uncertain effects <strong>of</strong> rosiglitazone on the risk for<br />
myocardial infarction and cardiovascular death. Ann Intern Med. 2007;147:578-581.<br />
Home PD, Pocock SJ, Beck-Nielsen H, et al, for the RECORD Study Group.<br />
Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med.<br />
2007;357:28-38.<br />
Krall RL. <strong>Cardiovascular</strong> safety <strong>of</strong> rosiglitazone. Lancet. 2007;369:1995-1996.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 15 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Lago RM, Singh PP, Nes<strong>to</strong> RW. Congestive heart failure and cardiovascular death in<br />
patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis <strong>of</strong><br />
randomised clinical trials. Lancet. 2007;370:1129-1136.<br />
McAfee AT, Koro C, Landon J, Ziyadeh N, Walker AM. Coronary heart disease<br />
outcomes in patients receiving antidiabetic agents. Pharmacoepidemiol Drug Saf.<br />
2007;16:711-725.<br />
Nissen SE, Wolski K. Effect <strong>of</strong> rosiglitazone on the risk <strong>of</strong> myocardial infarction and<br />
death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.<br />
Rosen CJ. The rosiglitazone s<strong>to</strong>ry—lessons from an FDA Advisory Committee meeting.<br />
N Engl J Med. 2007;357:844-846.<br />
PROactive: Time <strong>to</strong> Primary Composite<br />
Endpoint: Pioglitazone vs. Placebo<br />
Kaplan-Meier Event Rate<br />
0.25<br />
0.20<br />
N events:<br />
Placebo 572 / 2633<br />
Pioglitazone 514 / 2605<br />
0.15 What if this was a<br />
3-Year Estimate:<br />
6-month trial ?<br />
23.5%<br />
0.10<br />
21.0%<br />
HR 95% CI p value<br />
0.05<br />
Pioglitazone<br />
0.80<br />
0.90<br />
vs. placebo<br />
1.02<br />
0.095<br />
0.0<br />
0 6 12 18 24 30 36<br />
No. at Risk: 5238 5018 4786 4619 4433 4268 693<br />
Time from Randomization (Months)<br />
CI = confidence interval; HR = hazard ratio<br />
Reprinted from Dormandy JA, et al. Lancet. 2005;366:<br />
1279–1289, with permission from Elsevier.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
PROactive: Time <strong>to</strong> Primary Composite Endpoint: Pioglitazone vs. Placebo<br />
The Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive)<br />
evaluated patients with type 2 diabetes and a his<strong>to</strong>ry <strong>of</strong> cardiovascular disease and<br />
randomized them <strong>to</strong> receive either pioglitazone or placebo. The primary endpoint showed<br />
a nonstatistical benefit in favor <strong>of</strong> pioglitazone.<br />
Reference:<br />
Dormandy JA, Charbonnel B, Eckland DJ, et al, for the PROactive investiga<strong>to</strong>rs.<br />
Secondary prevention <strong>of</strong> macrovascular events in patients with type 2 diabetes in the<br />
PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a<br />
randomised controlled trial. Lancet. 2005;366:1279-1289.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 16 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
PROactive: Difference in Number <strong>of</strong> First Events<br />
Comprising the Primary Composite Endpoint:<br />
Pioglitazone vs. Placebo<br />
Difference in Number <strong>of</strong><br />
First Events<br />
20<br />
10<br />
0<br />
-10<br />
-20<br />
-12<br />
Pioglitazone: n = 2605<br />
Placebo: n = 2633<br />
-10<br />
Death Nonfatal Silent Stroke MLA ACS Revascularization<br />
MI* MI coronary leg<br />
ACS = acute coronary syndrome; MI = myocardial infarction; MLA = major leg<br />
amputation<br />
Dormandy JA, et al. Lancet. 2005;366:1279–1289.<br />
-3<br />
*Excluding silent myocardial infarction<br />
-6<br />
-20 -21<br />
0<br />
+14<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
PROactive: Difference in Number <strong>of</strong> First Events Comprising the Primary<br />
Composite Endpoint: Pioglitazone vs. Placebo<br />
The primary endpoint shown on this slide contained a variety <strong>of</strong> cardiovascular<br />
outcomes, including death, myocardial infarction, stroke, acute coronary syndromes, and<br />
revascularization <strong>of</strong> either the coronary arteries or the peripheral arteries. Overall, the<br />
more “traditional” cardiovascular endpoints occurred less <strong>of</strong>ten in the pioglitazone group;<br />
however, this group had more coronary leg revascularizations than did the placebo group.<br />
Reference:<br />
Dormandy JA, Charbonnel B, Eckland DJ, et al, for the PROactive investiga<strong>to</strong>rs.<br />
Secondary prevention <strong>of</strong> macrovascular events in patients with type 2 diabetes in the<br />
PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a<br />
randomised controlled trial. Lancet. 2005;366:1279-1289.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 17 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
PROactive: Pioglitazone Reduces “Hard”<br />
Coronary Heart <strong>Disease</strong> Endpoints<br />
Kaplan-Meier Event Rate<br />
Time <strong>to</strong> Fatal/Nonfatal MI<br />
Time <strong>to</strong> Acute Coronary<br />
(Excluding Silent MI)<br />
Syndrome<br />
0.10<br />
HR 95% CI P value<br />
0.06<br />
HR 95% CI P value<br />
PIO vs.<br />
0.52<br />
PIO vs.<br />
0.41<br />
0.72<br />
.045<br />
0.63<br />
.035<br />
placebo<br />
0.99<br />
placebo<br />
0.97<br />
0.08<br />
0.05<br />
0.04<br />
0.06<br />
-28% -37%<br />
0.03<br />
0.04<br />
0.02<br />
0.02<br />
PIO (65/1230)<br />
PIO (35/1230)<br />
0.01<br />
Placebo (88/1215)<br />
Placebo (54/1215)<br />
0.00<br />
0.00<br />
0 6 12 18 24 30 36<br />
0 6 12 18 24 30 36<br />
Kaplan-Meier Event Rate<br />
Time from Randomization (Months)<br />
Time from Randomization (Months)<br />
Prespecified<br />
Post-Hoc Explora<strong>to</strong>ry Analysis<br />
CHD = coronary heart disease; CI = confidence interval; HR = hazard ratio; MI =<br />
myocardial infarction; PIO = pioglitazone<br />
Reprinted from Erdmann E, et al. J Am Coll Cardiol. 2007;49:1772-1780,<br />
with permission from Elsevier | Erdmann E. PROactiveresults.com. Available<br />
at: http://www.proactive-results.com/html/analysis.htm<br />
results.com/html/analysis.htm. © On Screen<br />
Productions Ltd. All rights reserved.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
PROactive: Pioglitazone Reduces “Hard” Coronary Heart <strong>Disease</strong> Endpoints<br />
In a prespecified secondary endpoint study in the PROactive trial, which examined time<br />
<strong>to</strong> fatal or nonfatal myocardial infarction (MI), patients who were treated with<br />
pioglitazone had a 28% reduction in MIs when compared <strong>to</strong> those who were treated with<br />
placebo. In a post-hoc explora<strong>to</strong>ry analysis, the pioglitazone group also benefited, with a<br />
reduction in the time <strong>to</strong> occurrence <strong>of</strong> acute coronary syndromes. These data would<br />
indicate that pioglitazone is not associated with an excess risk for MI, although the drug<br />
did increase the risk for congestive heart failure (CHF) in this trial. The increase in CHF<br />
risk is not surprising, since these patients had cardiovascular disease; many <strong>of</strong> them were<br />
also on insulin therapy and were randomized <strong>to</strong> receive pioglitazone at the highest dosage<br />
range.<br />
References:<br />
Erdmann E, for the PROactive Investiga<strong>to</strong>rs. The effect <strong>of</strong> pioglitazone on recurrent<br />
myocardial infarction in 2445 patients with type 2 diabetes and previous myocardial<br />
infarction: results from the PROactive study. PROactiveresults.com.<br />
http://www.proactive-results.com/html/analysis.htm. Accessed November 2008.<br />
Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM,<br />
for the PROactive Investiga<strong>to</strong>rs. The effect <strong>of</strong> pioglitazone on recurrent myocardial<br />
infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction:<br />
results from the PROactive (PROactive 05) Study. J Am Coll Cardiol. 2007;49:1772-<br />
1780.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 18 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Fac<strong>to</strong>rs Possibly Contributing <strong>to</strong> the<br />
Beneficial Effect <strong>of</strong> Pioglitazone<br />
Hemoglobin A 1c <br />
−0.5%<br />
HDL cholesterol +8.9%<br />
Blood pressure <br />
−3 mm Hg<br />
LDL/HDL 5.3%<br />
Triglycerides <br />
−13.2%<br />
vs. placebo<br />
C-reactive protein<br />
Insulin sensitivity<br />
Not measured<br />
Not measured<br />
HDL = high-density lipoprotein; LDL = low-density lipoprotein<br />
Reprinted from Dormandy JA, et al. Lancet 2005;366:<br />
1279–1289, with permission from Elsevier.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Fac<strong>to</strong>rs Possibly Contributing <strong>to</strong> the Beneficial Effect <strong>of</strong> Pioglitazone<br />
The fac<strong>to</strong>rs possibly contributing <strong>to</strong> the beneficial effect <strong>of</strong> pioglitazone include: a<br />
modest reduction <strong>of</strong> 0.5% in the concentration <strong>of</strong> hemoglobin A 1c , an increase <strong>of</strong><br />
approximately 9% in the concentration <strong>of</strong> high-density lipoprotein (HDL), a drop <strong>of</strong> 3<br />
mm Hg in blood pressure, an improvement in the low-density lipoprotein (LDL):HDL<br />
ratio, and a drop in the concentration <strong>of</strong> triglycerides. The aggregate directional change in<br />
these fac<strong>to</strong>rs may have accounted for the benefit <strong>of</strong> pioglitazone when compared <strong>to</strong><br />
placebo in the PROactive trial, as mentioned earlier.<br />
Reference:<br />
Dormandy JA, Charbonnel B, Eckland DJ, et al, for the PROactive investiga<strong>to</strong>rs.<br />
Secondary prevention <strong>of</strong> macrovascular events in patients with type 2 diabetes in the<br />
PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a<br />
randomised controlled trial. Lancet. 2005;366:1279-1289.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 19 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Risk <strong>of</strong> Congestive Heart Failure and <strong>Cardiovascular</strong><br />
Death With Thiazolidinediones<br />
Risk <strong>of</strong> CHF<br />
ADOPT (RSG)<br />
Dargie et al. (RSG)<br />
Mazzone et al. (Pio)<br />
DREAM (RSG)<br />
PPAR (RSG)<br />
PROactive (Pio)<br />
RECORD (RSG)<br />
TOTAL<br />
Risk <strong>of</strong> CHF<br />
ADOPT (RSG)<br />
Dargie et al. (RSG)<br />
Mazzone et al. (Pio)<br />
DREAM (RSG)<br />
PPAR (RSG)<br />
PROactive (Pio)<br />
RECORD (RSG)<br />
TOTAL<br />
Risk Ratio (95% Confidence Interval)<br />
Decreased Risk Increased Risk<br />
0.1 0.2 0.5 1 2 5 10<br />
Risk Ratio (95% Confidence Interval)<br />
Decreased Risk Increased Risk<br />
0.1 0.2 0.5 1 2 5 10<br />
Weight<br />
12.0%<br />
7.3%<br />
1.1%<br />
5.0%<br />
1.2%<br />
49.0%<br />
23.5%<br />
100.0%<br />
Weight<br />
9.8%<br />
6.4%<br />
1.4%<br />
15.2%<br />
1.9%<br />
20.9%<br />
44.5%<br />
100.0%<br />
Risk Ratio (95% CI)<br />
1.49 (0.62, 3.53)<br />
1.81 (0.55, 6.02)<br />
2.97 (0.12, 72.63)<br />
7.00 (1.59, 30.76)<br />
2.88 (0.12, 69.94)<br />
1.31 (1.03, 1.67)<br />
2.24 (1.27, 3.96)<br />
1.72 (1.21, 2.42)<br />
Test for overall effect: p = 0.002<br />
Risk Ratio (95% CI)<br />
0.83 (0.29, 2.35)<br />
1.30 (0.36, 4.07)<br />
0.99 (0.06, 15.75)<br />
1.20 (0.52, 2.77)<br />
0.48 (0.04, 5.21)<br />
1.01 (0.50, 2.06)<br />
0.83 (0.51, 1.35)<br />
0.93 (0.67, 1.29)<br />
Test for overall effect: p = 0.068<br />
Reprinted from Lago RM, et al. Lancet. 2007;<br />
370:1129–1136, with permission from Elsevier.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Risk <strong>of</strong> Congestive Heart Failure and <strong>Cardiovascular</strong> Death With<br />
Thiazolidinediones<br />
A meta-analysis by Lago et al. (2007) examined randomized clinical trials in which<br />
cardiovascular (CV) events were either endpoints or were adjudicated by an expert panel.<br />
These investiga<strong>to</strong>rs found that there was no excess risk <strong>of</strong> CV-related death when<br />
thiazolidinediones were compared <strong>to</strong> other treatments and when rosiglitazone was<br />
compared <strong>to</strong> pioglitazone.<br />
Reference:<br />
Lago RM, Singh PP, Nes<strong>to</strong> RW. Congestive heart failure and cardiovascular death in<br />
patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis <strong>of</strong><br />
randomised clinical trials. Lancet. 2007;370:1129-1136.<br />
Annual Incidence <strong>of</strong> Congestive Heart Failure in<br />
Randomized Clinical Trials <strong>of</strong> PPAR- Agonists<br />
Trial<br />
<strong>Disease</strong><br />
N<br />
Diagnosed CHF<br />
TZD<br />
Compara<strong>to</strong>r<br />
DREAM<br />
IFG /IGT<br />
5269<br />
Adjudicated<br />
RSG 0.18%<br />
Placebo 0.03%<br />
ADOPT<br />
T2DM<br />
4351<br />
Adverse events<br />
RSG 0.21%<br />
SU 0.5%<br />
Metformin 0.21%<br />
RECORD<br />
T2DM<br />
4447<br />
Hospital CHF,<br />
adjudicated, and<br />
pending<br />
RSG 0.56%<br />
Metformin / SU<br />
0.26%<br />
CHICAGO<br />
T2DM<br />
462<br />
Adjudicated CHF<br />
Pio 0.62%<br />
SU (0)<br />
PPAR<br />
Metabolic Syndrome<br />
CAD + PCI<br />
200<br />
Adjudicated CHF<br />
RSG 1.2%<br />
Placebo (0)<br />
PROactive<br />
T2DM / CVD<br />
5238<br />
Hospitalized<br />
Pio 2.0%<br />
Placebo 1.4%<br />
PROactive<br />
T2DM / CVD Status<br />
Post MI<br />
2445<br />
Hospitalized<br />
Pio 2.6%<br />
Placebo 1.8%<br />
Dargie et al.<br />
T2DM / NYHA I/II CHF<br />
224<br />
Adjudicated CHF<br />
RSG 6.3%<br />
Placebo 3.5%<br />
CAD = coronary artery disease; CHF= congestive heart failure; CVD = cardiovascular disease; IFG =<br />
impaired fasting glucose; IGT = impaired glucose <strong>to</strong>lerance; MI = myocardial infarction; NYHA = New<br />
York Heart Association; PCI = percutaneous coronary intervention; Pio = pioglitazone; PPAR =<br />
peroxisome prolifera<strong>to</strong>r-activated recep<strong>to</strong>r; RSG = rosiglitazone; SU = sulfonylurea; T2DM = type 2<br />
diabetes mellitus; TZD = thiazolidinedione<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 20 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
Annual Incidence <strong>of</strong> Congestive Heart Failure in Randomized Clinical Trials <strong>of</strong><br />
PPAR- Agonists<br />
The major problem associated with the use <strong>of</strong> thiazolidinediones, in general, has been the<br />
risk <strong>of</strong> congestive heart failure (CHF). This risk varies, depending on whether the patient<br />
has pre-existing cardiovascular disease. As one can see in this slide, which summarizes a<br />
large number <strong>of</strong> randomized clinical trials, the annual incidence <strong>of</strong> CHF varies greatly.<br />
For example, the annual risk <strong>of</strong> CHF was 0.21% per year in the ADOPT trial, and CHFrelated<br />
hospitalization occurred annually in 2.00% <strong>of</strong> the patients in the PROactive trial.<br />
PROactive: Risk Fac<strong>to</strong>rs for Serious<br />
Congestive Heart Failure<br />
Creatinine 130 µmol/L<br />
Diuretic use<br />
LDL cholesterol >4 mmol/L<br />
(versus
Clinical Trials Will (or Should) Answer<br />
Important Questions About Type 2 Diabetes<br />
Mellitus and <strong>Cardiovascular</strong> Risk<br />
Question 1:<br />
Does treatment-directed<br />
lowering <strong>of</strong> HbA 1c 1c<br />
reduce<br />
the risk <strong>of</strong> cardiovascular<br />
disease?<br />
• ACCORD<br />
• ADVANCE<br />
• VADT<br />
• ORIGIN<br />
Question 2:<br />
Does it matter which<br />
diabetes treatment is used<br />
<strong>to</strong> achieve a lower HbA 1c 1c<br />
concentration?<br />
• PERISCOPE<br />
• APPROACH<br />
• BARI-2D<br />
• RECORD<br />
• VICTORY<br />
HbA 1c = hemoglobin A 1c<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Clinical Trials Will (or Should) Answer Important Questions About Type 2<br />
Diabetes Mellitus and <strong>Cardiovascular</strong> Risk<br />
There are two major questions regarding type 2 diabetes and cardiovascular risk. First,<br />
does treatment-directed lowering <strong>of</strong> the hemoglobin A 1c concentration reduce the risk <strong>of</strong><br />
cardiovascular disease? We have received some answers <strong>to</strong> this question from the<br />
findings <strong>of</strong> the recent ACCORD, ADVANCE, and VADT studies. The ORIGIN trial is<br />
underway and is evaluating whether giving insulin <strong>to</strong> patients with prediabetes or newonset<br />
diabetes can reduce cardiovascular disease.<br />
Second, does it matter which diabetes treatment is used <strong>to</strong> achieve a lower concentration<br />
<strong>of</strong> hemoglobin A 1c ? The PERIOSCOPE and APPROACH trials each looked at the<br />
progression <strong>of</strong> atherosclerosis by using intracoronary ultrasound <strong>to</strong> compare the effects <strong>of</strong><br />
treatment with thiazolidinediones or sulfonylureas. The BARI-2D trial is evaluating<br />
whether an insulin-sensitizing approach is better than an insulin-providing approach in<br />
reducing cardiovascular events in patients with type 2 diabetes and coronary artery<br />
disease. The RECORD trial is comparing rosiglitazone <strong>to</strong> sulfonylureas and metformin<br />
with regard <strong>to</strong> cardiovascular endpoints, and the VICTORY trial is examining the<br />
progression <strong>of</strong> disease in saphenous vein grafts in diabetic patients who were randomized<br />
<strong>to</strong> receive rosiglitazone or placebo.<br />
ACCORD = Action <strong>to</strong> Control <strong>Cardiovascular</strong> Risk in Diabetes<br />
ADVANCE = Action in Diabetes and Vascular <strong>Disease</strong>: Preterax and Diamicron MR<br />
Controlled Evaluation<br />
APPROACH = Assessment on the Prevention <strong>of</strong> Progression by Rosiglitazone on<br />
Atherosclerosis in diabetes patients with <strong>Cardiovascular</strong> His<strong>to</strong>ry<br />
BARI-2D = Bypass Angioplasty Revascularization Investigations 2 Diabetes<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 22 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
ORIGIN = Outcome Reduction with Initial Glargine Intervention<br />
PERISCOPE = Pioglitazone Effect on Regression <strong>of</strong> Intravascular Sonographic Coronary<br />
Obstruction Prospective Evaluation<br />
RECORD = Rosiglitazone Evaluated for Cardiac Outcomes and Regulation <strong>of</strong> <strong>Glycemia</strong><br />
in Diabetes<br />
VADT = Veterans Affairs Diabetes Trial<br />
VICTORY = Vein-Coronary Atherosclerosis and Rosiglitazone After Bypass Surgery<br />
ACCORD: Deaths in Intensive vs. Standard<br />
Glycemic Control Groups: Preliminary Results<br />
Deaths, n<br />
Rate per 1,000<br />
patients/year<br />
Standard Glycemic Control<br />
(Hemoglobin A 1c 7.0–7.9%)<br />
203<br />
11<br />
Intensive Glycemic<br />
Control<br />
(Hemoglobin A 1c ,
http://www.nhlbi.nih.gov/health/pr<strong>of</strong>/heart/other/accord/remarks.pdf. Dated February 6,<br />
2008. Accessed November 2008.<br />
National Heart Lung and Blood Institute. For safety, NHLBI changes intensive blood<br />
sugar treatment strategy in clinical trial <strong>of</strong> diabetes and cardiovascular disease. National<br />
Heart Lung and Blood Institute Web site. http://www.nih.gov/news/health/feb2008/nhlbi-<br />
06.htm. Dated February 6, 2008. Accessed November 2008.<br />
ADVANCE: Action in Diabetes and Vascular <strong>Disease</strong>:<br />
Preterax and Diamicron MR Controlled Evaluation<br />
Primary objective:<br />
To determine the effect <strong>of</strong> blood pressure treatment (ACE-inhibi<strong>to</strong>r<br />
+ diuretic vs. placebo) and glycemic intervention (intensive vs.<br />
standard) on microvascular and macrovascular complications in<br />
patients with type 2 diabetes mellitus (2 2 fac<strong>to</strong>rial design)<br />
Patient population: Type 2 diabetes (hypertensive + nonhypertensive) (N = 11,140)<br />
Glycemic Control<br />
Blood Pressure Intervention<br />
Intensive* (HbA 1c ≤6.5%) Perindopril + Indapamide<br />
Placebo<br />
Standard <br />
Perindopril + Indapamide<br />
Placebo<br />
*Gliclazide plus oral agents/insulin <strong>to</strong> achieve hemoglobin A 1c (HbA 1c ) target<br />
Based on standard guidelines<br />
Primary endpoint:<br />
Macrovascular: composite <strong>of</strong> nonfatal stroke, nonfatal<br />
myocardial infarction, cardiovascular death<br />
Microvascular: composite <strong>of</strong> new or worsening nephropathy<br />
or retinopathy<br />
Secondary endpoints: Cerebrovascular disease, cardiovascular disease, peripheral<br />
vascular disease, microalbuminuria, visual deterioration,<br />
neuropathy, heart failure, cognitive function and dementia,<br />
all-cause mortality<br />
Treatment duration:<br />
4.5 years<br />
ADVANCE Management Committee. Diabe<strong>to</strong>logia. 2001;44:1118–1120.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
ADVANCE: Action in Diabetes and Vascular <strong>Disease</strong>: Preterax and Diamicron MR<br />
Controlled Evaluation<br />
The ADVANCE trial showed that lowering glucose with a target <strong>of</strong>
PERISCOPE: Study Design<br />
IVUS at<br />
Screening<br />
Visit<br />
IVUS = intravascular<br />
ultrasound<br />
Randomization<br />
Pioglitazone<br />
18 Months<br />
Glimepiride<br />
IVUS at<br />
Final<br />
Visit<br />
• Pioglitazone HCl (15 mg titrated <strong>to</strong> 30 mg titrated <strong>to</strong> 45 mg or 30 mg titrated<br />
<strong>to</strong> 45 mg, depending on the dose <strong>of</strong> sulfonylurea)<br />
• Glimepiride (1 mg titrated <strong>to</strong> 2 mg titrated <strong>to</strong> 4 mg or 2 mg titrated <strong>to</strong> 4 mg,<br />
depending on the dose <strong>of</strong> sulfonylurea)<br />
• Allowance <strong>to</strong> increase the standard <strong>of</strong> therapy once on maximum dose <strong>of</strong><br />
study medication, if required <strong>to</strong> maintain glycemic control<br />
• Allowance <strong>to</strong> decrease the standard <strong>of</strong> therapy in order <strong>to</strong> maintain adequate<br />
glycemic control<br />
Adapted from Nissen SE, et al. JAMA. 2008;299:1561–1573.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
PERISCOPE: Study Design<br />
The recent findings <strong>of</strong> the PERISCOPE study showed that, among diabetic patients<br />
undergoing coronary angiography, those treated with pioglitazone had less progression <strong>of</strong><br />
atherosclerosis as measured by intravascular (intracoronary) ultrasound when compared<br />
<strong>to</strong> patients who were treated with glimepiride.<br />
PERISCOPE = Pioglitazone Effect on Regression <strong>of</strong> Intravascular Sonographic Coronary<br />
Obstruction Prospective Evaluation<br />
Reference:<br />
Nissen SE, Nicholls SJ, Wolski K, et al, for the PERISCOPE Investiga<strong>to</strong>rs. Comparison<br />
<strong>of</strong> pioglitazone vs. glimepiride on progression <strong>of</strong> coronary atherosclerosis in patients with<br />
type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561-<br />
1573.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 25 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
PERISCOPE: Primary Efficacy Parameter:<br />
Change in Percent Atheroma Volume<br />
Change in Percent<br />
Atheroma Volume (%)<br />
0.9<br />
0.7<br />
0.5<br />
0.3<br />
0.1<br />
-0.1<br />
-0.3<br />
p < 0.001<br />
0.73<br />
Glimepiride<br />
Glimepiride (n = 181)<br />
Pioglitazone (n = 179)<br />
P = 0.002<br />
−0.16<br />
p = 0.44<br />
Pioglitazone<br />
Nissen SE, et al. JAMA. 2008;299:1561–1573.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
PERISCOPE: Primary Efficacy Parameter: Change in Percent Atheroma Volume<br />
The primary efficacy parameter in the PERISCOPE trial was the change in percent<br />
atheroma volume. Patients treated with glimepiride had an increase in percent atheroma<br />
volume over the 18 months between coronary assessments, whereas those treated with<br />
pioglitazone had no such increase.<br />
PERISCOPE = Pioglitazone Effect on Regression <strong>of</strong> Intravascular Sonographic Coronary<br />
Obstruction Prospective Evaluation<br />
Reference:<br />
Nissen SE, Nicholls SJ, Wolski K, et al, for the PERISCOPE Investiga<strong>to</strong>rs. Comparison<br />
<strong>of</strong> pioglitazone vs. glimepiride on progression <strong>of</strong> coronary atherosclerosis in patients with<br />
type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561-<br />
1573.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 26 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
PERISCOPE: Intravascular<br />
Ultrasound: Secondary Endpoints<br />
0.015<br />
Atheroma<br />
Thickness (mm)<br />
0.0<br />
Atheroma<br />
Volume (mm 3 )<br />
0.0<br />
Most-<strong>Disease</strong>d<br />
10 mm (mm 3 )<br />
0.010<br />
0.011<br />
-1.0<br />
-1.5<br />
0.005<br />
P = 0.006<br />
-2.0<br />
-1.0<br />
P = 0.06 P = 0.93<br />
0.000<br />
-3.0<br />
-0.005<br />
-4.0<br />
-2.0<br />
-2.1<br />
-2.0<br />
-0.010<br />
-0.011<br />
-5.0<br />
-5.5<br />
-0.015<br />
-6.0<br />
Glimepiride<br />
-3.0<br />
Pioglitazone<br />
Nissen SE, et al. JAMA. 2008;299:1561–1573.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
PERISCOPE: Intravascular Ultrasound: Secondary Endpoints<br />
Furthermore, atheroma thickness, atheroma volume, and the severity <strong>of</strong> the most diseased<br />
10-mm segment – in addition <strong>to</strong> percent atheroma volume – were examined in the<br />
PERISCOPE trial. All <strong>of</strong> these secondary endpoints showed trends <strong>to</strong>ward benefit in the<br />
group treated with pioglitazone.<br />
PERISCOPE = Pioglitazone Effect on Regression <strong>of</strong> Intravascular Sonographic Coronary<br />
Obstruction Prospective Evaluation<br />
Reference:<br />
Nissen SE, Nicholls SJ, Wolski K, et al, for the PERISCOPE Investiga<strong>to</strong>rs. Comparison<br />
<strong>of</strong> pioglitazone vs. glimepiride on progression <strong>of</strong> coronary atherosclerosis in patients with<br />
type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561-<br />
1573.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 27 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
IVUS as a Tool <strong>to</strong> Evaluate Coronary<br />
Atherosclerosis: Change from Baseline<br />
in Percent Atheroma Volume<br />
APO = apolipoprotein; IVUS = intravascular ultrasound;<br />
NS = not significant; PAV = percent atheroma volume<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
IVUS as a Tool <strong>to</strong> Evaluate Coronary Atherosclerosis: Change from Baseline in<br />
Percent Atheroma Volume<br />
When one places the results <strong>of</strong> the PERISCOPE trial in the context <strong>of</strong> several other trials<br />
using this methodology (i.e., intravascular ultrasound) <strong>to</strong> evaluate atherosclerosis in<br />
response <strong>to</strong> various interventions, the benefit <strong>of</strong> pioglitazone (when compared <strong>to</strong><br />
glimepiride) was <strong>of</strong> approximately the same magnitude as other interventions in reducing<br />
atheroma progression.<br />
ASTEROID = A Study <strong>to</strong> Evaluate the Effect <strong>of</strong> Rosuvastatin on Intravascular<br />
Ultrasound<br />
CAMELOT = Comparison <strong>of</strong> Amlodipine vs. Enalapril <strong>to</strong> Limit Occurrences <strong>of</strong><br />
Thrombosis<br />
IVUS = intravascular ultrasound<br />
PERISCOPE = Pioglitazone Effect on Regression <strong>of</strong> Intravascular Sonographic Coronary<br />
Obstruction Prospective Evaluation<br />
REVERSAL = REVERSing Atherosclerosis with Aggressive Lipid Lowering<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 28 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
APPROACH: Using Intravascular Ultrasound <strong>to</strong> Assess<br />
Progression <strong>of</strong> Coronary Artery <strong>Disease</strong> With Rosiglitazone<br />
• Population:<br />
– 600 patients with type 2 diabetes and coronary artery disease admitted for<br />
angiography or percutaneous coronary intervention<br />
• Intervention:<br />
– Rosiglitazone vs. control with comparable glycemic control (Glipizide)<br />
– Adherence <strong>to</strong> cardiovascular risk fac<strong>to</strong>r guidelines<br />
• Endpoints:<br />
– 1% change in percent atheroma volume within a 40-mm segment <strong>of</strong><br />
nonintervened coronary artery based on intravascular ultrasound<br />
Patients with T2DM<br />
and CAD <strong>to</strong> Have PCI<br />
or Angiography<br />
n = 300, Active Arm - Rosiglitazone<br />
n = 300, Control Arm - Glipizide<br />
Randomization<br />
Screen <br />
IVUS and Angiography<br />
18-Month Treatment<br />
<br />
Week<br />
- 1 0 1 2 3 6 9 12 15 18<br />
CAD = coronary artery disease; IVUS = intravascular ultrasound;<br />
PCI = percutaneous coronary intervention; T2DM = type 2 diabetes mellitus<br />
Ratner RE, et al. Am Heart J 2008;156:1074−1079.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
APPROACH: Using Intravascular Ultrasound <strong>to</strong> Assess Progression <strong>of</strong> Coronary<br />
Artery <strong>Disease</strong> With Rosiglitazone<br />
The APPROACH trial, which was reported at the annual scientific sessions <strong>of</strong> American<br />
Heart Association in November 2008, has a study method similar <strong>to</strong> that <strong>of</strong> the<br />
PERISCOPE trial. Patients with diabetes and coronary artery disease undergoing<br />
coronary angiography were randomized <strong>to</strong> receive either rosiglitazone or glipizide.<br />
Patients had assessments <strong>of</strong> percent atheroma volume at baseline and at the end <strong>of</strong> the 18-<br />
month treatment phase.<br />
APPROACH = Assessment on the Prevention <strong>of</strong> Progression by Rosiglitazone on<br />
Atherosclerosis in diabetes patients with <strong>Cardiovascular</strong> His<strong>to</strong>ry<br />
Reference:<br />
Ratner RE, Cannon CP, Gerstein HC, et al, for the APPROACH Study Group.<br />
Assessment on the Prevention <strong>of</strong> Progression by Rosiglitazone on Atherosclerosis in<br />
diabetes patients with <strong>Cardiovascular</strong> His<strong>to</strong>ry (APPROACH): study design and baseline<br />
characteristics. Am Heart J. 2008;156:1074-1079.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 29 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
APPROACH: Primary Endpoint:<br />
Change in Percent Atheroma Volume<br />
Change in Percent<br />
Atheroma Volume (%)<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
0.0<br />
-0.1<br />
-0.2<br />
-0.3<br />
0.43 (−0.22,(<br />
1.08) P = 0.19<br />
Glipizide<br />
N = 462<br />
Treatment Difference<br />
−0.64 (−1.46,(<br />
0.17)<br />
P = 0.12<br />
Rosiglitazone<br />
−0.21 (−0.86,(<br />
0.44) P = 0.53<br />
Nes<strong>to</strong> RW, et al, for the APPROACH Study Group.<br />
Circulation. 2008;118:2313. Abstract #5221.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
APPROACH: Primary Endpoint: Change in Percent Atheroma Volume<br />
The primary endpoint, change in percent atheroma volume, was −0.21% in the<br />
rosiglitazone group versus 0.43% in the glipizide group (p = 0.12 between groups).<br />
By looking at the results <strong>of</strong> the PERISCOPE trial and the APPROACH trial <strong>to</strong>gether, and<br />
evaluating the relative benefits <strong>of</strong> thiazolidinediones against sulfonylureas with regard <strong>to</strong><br />
progression <strong>of</strong> atherosclerosis, a virtual “head-<strong>to</strong>-head” comparison <strong>of</strong> these two agents<br />
was made. The resulting data were interesting in view <strong>of</strong> the debate over the past year <strong>of</strong><br />
whether rosiglitazone and pioglitazone have different properties that might either increase<br />
or reduce the risk <strong>of</strong> cardiovascular events.<br />
APPROACH = Assessment on the Prevention <strong>of</strong> Progression by Rosiglitazone on<br />
Atherosclerosis in diabetes patients with <strong>Cardiovascular</strong> His<strong>to</strong>ry<br />
Reference:<br />
Nes<strong>to</strong> RW, Cannon CP, Gerstein HC, et al, for the APPROACH Study Group. Results <strong>of</strong><br />
the APPROACH trial: assessment on the prevention <strong>of</strong> progression by rosiglitazone on<br />
atherosclerosis in type 2 diabetes patients with cardiovascular his<strong>to</strong>ry. Circulation.<br />
2008;118:2313. Abstract #5221.<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 30 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
STENO-2:<br />
Reduction in <strong>Cardiovascular</strong> <strong>Disease</strong><br />
Through a Multifac<strong>to</strong>rial Intervention in Patients<br />
Who Have Type 2 Diabetes and Microalbuminuria<br />
Primary Composite<br />
Endpoint (%)<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
Steno Diabetes Center<br />
P = 0.007<br />
Conventional therapy<br />
Intensive therapy<br />
n = 80<br />
n = 80<br />
0<br />
0<br />
12 24 36 48 60 72<br />
Months <strong>of</strong> Follow-up<br />
84 96<br />
Intensive Treatment Goals: hemoglobin A 1c ,
STENO-2:<br />
No Other Clinical Trial <strong>of</strong> Patients With Type 2<br />
Diabetes Has Shown Such a Dramatic Reduction in<br />
<strong>Cardiovascular</strong> Events With a Pharmacologic Intervention<br />
Number <strong>of</strong><br />
<strong>Cardiovascular</strong> Events<br />
40 Intensive Therapy<br />
35<br />
Conventional Therapy<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
Stroke<br />
Revascularization<br />
Death from<br />
<strong>Cardiovascular</strong><br />
Causes<br />
Myocardial<br />
Infarction<br />
Coronary<br />
Artery<br />
Bypass<br />
Graft<br />
Surgery<br />
Percutaneus<br />
Coronary<br />
Intervention<br />
Amputation<br />
Reprinted from Gaede P, et al. N Engl J Med. 2003;358:580−591.<br />
Copyright © 2003 Massachusetts Medical Society. All rights reserved.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
STENO-2: No Other Clinical Trial <strong>of</strong> Patients With Type 2 Diabetes Has Shown<br />
Such a Dramatic Reduction in <strong>Cardiovascular</strong> Events With a Pharmacologic<br />
Intervention<br />
The patients in the STENO-2 study had fewer deaths from cardiovascular disease, had<br />
fewer strokes and myocardial infarctions, and had less need for bypass surgery or<br />
angioplasty and revascularization or amputations when compared <strong>to</strong> the patients<br />
randomized <strong>to</strong> the conventional-therapy group.<br />
Reference:<br />
Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect <strong>of</strong> a multifac<strong>to</strong>rial<br />
intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.<br />
STENO-2:<br />
Risk-Fac<strong>to</strong>r Targets Attained at 7.8<br />
Years With an Intensive Treatment Program<br />
Patients (%)<br />
80 p < .001<br />
Intensive Therapy<br />
Conventional Therapy<br />
p = 0.19<br />
60<br />
p = 0.001<br />
A Message<br />
40<br />
<strong>to</strong><br />
ACCORD?<br />
p = 0.06<br />
20<br />
p = 0.21<br />
0<br />
Hemoglobin<br />
A 1c<br />
STENO-2: Risk-Fac<strong>to</strong>r Targets Attained at 7.8 Years With an Intensive Treatment<br />
Program<br />
Among the group that was randomized <strong>to</strong> receive intensive therapy in the STENO-2<br />
study, more patients had a <strong>to</strong>tal cholesterol <strong>of</strong>
Slide Source<br />
STENO-2:<br />
Lipid-Lowering Lowering Therapy<br />
Accounted for More Than 70% <strong>of</strong><br />
<strong>Cardiovascular</strong> Risk Reduction<br />
Percent <strong>of</strong> Total Calculated Risk<br />
Reduction in <strong>Cardiovascular</strong><br />
<strong>Disease</strong>-Related Events<br />
80<br />
60<br />
40<br />
20<br />
0<br />
<strong>Lipids</strong><br />
Analysis <strong>of</strong> STENO-2 data based on the risk<br />
engine from the United Kingdom Prospective<br />
Diabetes Study (UKPDS)<br />
Hemoglobin<br />
A 1c<br />
Sys<strong>to</strong>lic<br />
Blood<br />
Pressure<br />
Reprinted with permission from Gaede P, Pederson O. Diabetes.<br />
2004;53(Suppl 3):S39−S47. Copyright © 2004 American Diabetes Association.<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
STENO-2: Lipid-Lowering Therapy Accounted for More Than 70% <strong>of</strong><br />
<strong>Cardiovascular</strong> Risk Reduction<br />
A subsequent analysis <strong>of</strong> those fac<strong>to</strong>rs that could be responsible for the dramatic<br />
reduction in cardiovascular events in the STENO-2 study showed that reduction in <strong>to</strong>tal<br />
cholesterol accounted for approximately 70% <strong>of</strong> the overall benefit, with less <strong>of</strong> the<br />
overall benefit attributable <strong>to</strong> either improvement in glycemic control or reduction <strong>of</strong><br />
sys<strong>to</strong>lic blood pressure.<br />
Reference:<br />
Gaede P, Pedersen O. Intensive integrated therapy <strong>of</strong> type 2 diabetes: implications for<br />
long-term prognosis. Diabetes. 2004;53(Suppl 3):S39-S47.<br />
SANDS Randomized Trial: Effects <strong>of</strong> Lower LDL-<br />
Cholesterol and Blood-Pressure Targets on C-IMT C<br />
and Left Ventricular Mass in Patients With Diabetes<br />
• Standard group treated <strong>to</strong> target LDL = 100 mg/dL and SBP = 130 mm Hg<br />
• Intensive group treated <strong>to</strong> target LDL = 70 mg/dL and SBP = 115 mm Hg<br />
• Carotid intima-media thickness (C-IMT) and left ventricular mass (LVM)<br />
measured at baseline and at 18 and 36 months<br />
Patients (%)<br />
80<br />
60<br />
40<br />
20<br />
Decrease (Improved) No Change Increase (Worsened)<br />
Intima-Media Thickness<br />
Patients (%)<br />
80<br />
60<br />
40<br />
20<br />
Left Ventricular Mass Index<br />
0<br />
Standard<br />
Treatment<br />
(n = 230)<br />
Aggressive<br />
Treatment<br />
(n = 224)<br />
Reprinted from Howard BV, et al. JAMA 2008;299:1678−1689.<br />
Copyright © 2008 American Medical Association. All rights reserved.<br />
0<br />
Standard<br />
Treatment<br />
(n = 200)<br />
Aggressive<br />
Treatment<br />
(n = 202)<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 34 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
SANDS Randomized Trial: Effects <strong>of</strong> Lower LDL-Cholesterol and Blood-Pressure<br />
Targets on C-IMT and Left Ventricular Mass in Diabetes<br />
In the SANDS trial, Native Americans with type 2 diabetes underwent measurements <strong>of</strong><br />
carotid intima-media thickness (C-IMT) and left ventricular mass (LVM) at baseline, at<br />
18 months, and again at 36 months. One group, called the “standard” group, had their<br />
low-density lipoprotein (LDL) targeted <strong>to</strong> 100 mg/dL and their sys<strong>to</strong>lic blood pressure<br />
(SBP) targeted <strong>to</strong> 130 mm Hg. The second group, called the “intensive” group, had<br />
targets <strong>of</strong> 70 mg/dL and 115 mm Hg for LDL and SBP, respectively. Among the patients<br />
randomized <strong>to</strong> the intensive targets, fewer patients had a progression <strong>of</strong> C-IMT, and<br />
fewer patients had an increase in LVM. This study is interesting because it suggests that<br />
lowering LDL and SBP <strong>to</strong> targets beyond that which are currently recommended for<br />
these patients could yield additional cardiovascular benefit.<br />
Reference:<br />
Howard BV, Roman MJ, Devereux RB, et al. Effect <strong>of</strong> lower targets for blood pressure<br />
and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA.<br />
2008;299:1678-1689.<br />
BARI-2D:<br />
Evaluating Treatment Options for<br />
Patients With Type 2 Diabetes Mellitus and<br />
Coronary Artery <strong>Disease</strong><br />
Inclusion Criteria<br />
Type 2 DM<br />
Stable CAD<br />
Exclusion Criteria<br />
Manda<strong>to</strong>ry CABG<br />
–Unstable CAD<br />
–CAD extent<br />
–Left ventricular<br />
function<br />
Insulin<br />
Providing<br />
Insulin<br />
Sensitizing<br />
2 x 2 Fac<strong>to</strong>rial Design (n = 2600)<br />
Medical Rx<br />
Medical Rx<br />
CATH<br />
Revascularization<br />
<strong>of</strong> Choice<br />
and<br />
Medical Rx<br />
Revascularization<br />
<strong>of</strong> Choice<br />
and<br />
Medical Rx<br />
CABG = coronary artery bypass graft; CAD = coronary artery disease; DM = diabetes mellitus<br />
Brooks MM, et al. Am J Cardiol. 2006;97:9G-19G.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
BARI-2D: Evaluating Treatment Options for Patients With Type 2 Diabetes<br />
Mellitus and Coronary Artery <strong>Disease</strong><br />
In the trial design <strong>of</strong> the BARI-2D study, patients with diabetes and stable coronary<br />
artery disease are randomized <strong>to</strong> receive either an insulin-providing regimen with<br />
sulfonylurea and insulin or an insulin-sensitizing regimen with metformin and<br />
rosiglitazone. Patients are also randomized <strong>to</strong> revascularization <strong>of</strong> choice with either<br />
percutaneous coronary intervention or coronary artery bypass graft surgery, depending on<br />
the extent <strong>of</strong> coronary artery disease, or intensive medical treatment for angina. The<br />
results <strong>of</strong> this study will be reported at meetings <strong>of</strong> the American Diabetes Association in<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 35 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas
2009. This study should help <strong>to</strong> resolve the question about whether the manner in which<br />
diabetes is treated has an impact on cardiovascular outcomes.<br />
Reference:<br />
Brooks MM, Frye RL, Genuth S, et al, for the Bypass Angioplasty Revascularization<br />
Investigation 2 Diabetes (BARI 2D) Trial Investiga<strong>to</strong>rs. Hypotheses, design, and methods<br />
for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial.<br />
Am J Cardiol. 2006;97:9G-19G.<br />
Conclusion<br />
• Treatment <strong>of</strong> traditional risk fac<strong>to</strong>rs, such as<br />
blood pressure and lipids, reduces<br />
cardiovascular disease events in patients with<br />
diabetes.<br />
• However, even after treatment <strong>of</strong> traditional<br />
risk fac<strong>to</strong>rs, there remains an increased risk <strong>of</strong><br />
events in diabetic patients.<br />
• Ongoing trials will determine whether a<br />
treatment that is directed at lowering<br />
hemoglobin A 1c reduces the risk <strong>of</strong><br />
cardiovascular disease.<br />
• Ongoing trials will determine whether certain<br />
antidiabetic drugs can reduce the risk <strong>of</strong><br />
cardiovascular disease.<br />
Slide Source<br />
<strong>Lipids</strong> <strong>Online</strong> Slide Library<br />
www.lipidsonline.org<br />
Conclusion<br />
Source: <strong>Lipids</strong> <strong>Online</strong> Slide Library (www.lipidsonline.org) Page 36 <strong>of</strong> 36<br />
© 2009 Baylor College <strong>of</strong> Medicine, Hous<strong>to</strong>n, Texas