Navigating the tense, complex oncology market - Kantar Health
Navigating the tense, complex oncology market - Kantar Health
Navigating the tense, complex oncology market - Kantar Health
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
maY/JUNE 2012<br />
Brand Marketing & Communications<br />
<strong>Navigating</strong> <strong>the</strong> <strong>tense</strong>, <strong>complex</strong> <strong>oncology</strong><br />
<strong>market</strong><br />
Oncology combines<br />
some of <strong>the</strong> most lifechanging<br />
<strong>the</strong>rapeutic<br />
events with <strong>the</strong> most<br />
<strong>complex</strong> commercialization<br />
pathways.<br />
Rising costs of treatments<br />
simply add to<br />
<strong>the</strong> tension<br />
By Suzanne Shelley<br />
In early June, more than<br />
30,000 cancer specialists and<br />
more than 400 exhibitors are<br />
expected to ga<strong>the</strong>r in Chicago<br />
for this year’s American Soc.<br />
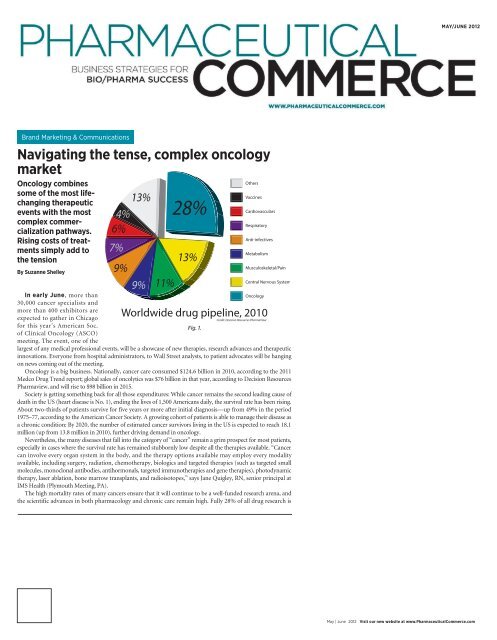
Fig. 1.<br />
of Clinical Oncology (ASCO)<br />
meeting. The event, one of <strong>the</strong><br />
largest of any medical professional events, will be a showcase of new <strong>the</strong>rapies, research advances and <strong>the</strong>rapeutic<br />
innovations. Everyone from hospital administrators, to Wall Street analysts, to patient advocates will be hanging<br />
on news coming out of <strong>the</strong> meeting.<br />
Oncology is a big business. Nationally, cancer care consumed $124.6 billion in 2010, according to <strong>the</strong> 2011<br />
Medco Drug Trend report; global sales of oncolytics was $76 billion in that year, according to Decision Resources<br />
Pharmaview, and will rise to $98 billion in 2015.<br />
Society is getting something back for all those expenditures: While cancer remains <strong>the</strong> second leading cause of<br />
death in <strong>the</strong> US (heart disease is No. 1), ending <strong>the</strong> lives of 1,500 Americans daily, <strong>the</strong> survival rate has been rising.<br />
About two-thirds of patients survive for five years or more after initial diagnosis—up from 49% in <strong>the</strong> period<br />
1975–77, according to <strong>the</strong> American Cancer Society. A growing cohort of patients is able to manage <strong>the</strong>ir disease as<br />
a chronic condition: By 2020, <strong>the</strong> number of estimated cancer survivors living in <strong>the</strong> US is expected to reach 18.1<br />
million (up from 13.8 million in 2010), fur<strong>the</strong>r driving demand in <strong>oncology</strong>.<br />
Never<strong>the</strong>less, <strong>the</strong> many diseases that fall into <strong>the</strong> category of “cancer” remain a grim prospect for most patients,<br />
especially in cases where <strong>the</strong> survival rate has remained stubbornly low despite all <strong>the</strong> <strong>the</strong>rapies available. “Cancer<br />
can involve every organ system in <strong>the</strong> body, and <strong>the</strong> <strong>the</strong>rapy options available may employ every modality<br />
available, including surgery, radiation, chemo<strong>the</strong>rapy, biologics and targeted <strong>the</strong>rapies (such as targeted small<br />
molecules, monoclonal antibodies, antihormonals, targeted immuno<strong>the</strong>rapies and gene <strong>the</strong>rapies), photodynamic<br />
<strong>the</strong>rapy, laser ablation, bone marrow transplants, and radioisotopes,” says Jane Quigley, RN, senior principal at<br />
IMS <strong>Health</strong> (Plymouth Meeting, PA).<br />
The high mortality rates of many cancers ensure that it will continue to be a well-funded research arena, and<br />
<strong>the</strong> scientific advances in both pharmacology and chronic care remain high. Fully 28% of all drug research is<br />
May | June 2012 Visit our new website at www.PharmaceuticalCommerce.com
Brand Marketing &<br />
Communications<br />
<strong>Navigating</strong> <strong>the</strong> <strong>tense</strong>, <strong>complex</strong> <strong>oncology</strong> <strong>market</strong><br />
targeted at cancer, according to Decision Resources (Fig. 1);<br />
PhRMA counted 887 drugs in Phase I–III development last<br />
year, including 108 for leukemia alone, 98 for lung cancer,<br />
and 91 for breast cancer. Key research directions include <strong>the</strong><br />
development of clinical endpoints (surrogates for identifying<br />
a positive outcome); alternative delivery routes (oral drugs vs.<br />
injectables); and combination and adjuvant <strong>the</strong>rapies (which<br />
combine multiple molecular entities as a single <strong>the</strong>rapy).<br />
The payer environment<br />
The growing list of commercialized cancer <strong>the</strong>rapies (Fig.<br />
2)—many of which represent a thrilling new lease on life<br />
for cancer patients—are <strong>the</strong> justification for <strong>the</strong> hundreds<br />
of additional drugs in development. But that commercial<br />
success creates its own problems: paying for <strong>the</strong> <strong>the</strong>rapies, and<br />
realigning <strong>the</strong> economics of how drugs are made available to<br />
patients. A 2009 study in <strong>the</strong> New England Journal of Medicine<br />
(NEJM, 2009, 360:626–633) found that <strong>the</strong> median cost of<br />
newly approved cancer medications had increased more than<br />
4.5 times in <strong>the</strong> preceding decade — up from $1,553 per month<br />
for <strong>the</strong> period 1995–1999 to $7,112 for 2005–2009 (constant<br />
2007 dollars). At <strong>the</strong> same time, <strong>oncology</strong> service providers<br />
are still adjusting to reductions in Medicare reimbursements<br />
instituted in 2005. Community-based oncologists, like most<br />
independent physician practices, are undergoing a wave of<br />
business realignments that connect <strong>the</strong>m (or outright sell<br />
<strong>the</strong>m) to hospital systems and integrated delivery networks.<br />
And that, in turn, affects how manufacturers can gain access<br />
to <strong>the</strong> <strong>market</strong>.<br />
“With <strong>the</strong> average cost of cancer care rising from $53,000<br />
per patient per year a decade ago to $150,000 per patient<br />
per year now, <strong>the</strong> trend is literally unsustainable,” says Burt<br />
Zweigenhaft, CEO of OncoMed (New York, NY), a specialty<br />
pharmacy. “We can no longer say we don’t care about<br />
formulary cost considerations or we don’t have to actually<br />
collect patient co-pays or carry out comparative-effectiveness<br />
studies.”<br />
Over <strong>the</strong> past few years, FDA has raised <strong>the</strong> bar for<br />
approval, and private and government payers have been<br />
more aggressive about scrutinizing <strong>the</strong>ir coverage policies<br />
related to <strong>oncology</strong> and instituting cost-control measures.<br />
These include stricter requirements for prior authorizations<br />
and adherence to FDA label indications, and limiting <strong>the</strong><br />
off-label use of anti-cancer treatments to Medicare-approved<br />
compendia listings. “With this heightened scrutiny, payers<br />
often have a higher expectation of clinically relevant benefit<br />
compared to <strong>the</strong> level of clinical response that is required<br />
for <strong>the</strong> drug to gain regulatory approval in <strong>the</strong> first place,”<br />
says Debbie Warner, VP, commercial planning, at <strong>Kantar</strong><br />
<strong>Health</strong> (Horsham, PA). “For instance, payers frequently cite<br />
a minimum of six-month improvement in overall survival<br />
before <strong>the</strong>y feel a treatment is ‘worth’ its cost. While approved<br />
drugs that deliver significantly lesser clinical benefit may still<br />
be covered, <strong>the</strong>y may face a much higher level of scrutiny in<br />
<strong>the</strong> prior authorization process to help control costs.”<br />
Concerns like <strong>the</strong>se hung up Provenge (sipuleucel-T), <strong>the</strong><br />
innovative cancer “vaccine” for prostate cancer, for its maker,<br />
Dendreon, in 2008–09, until FDA relented and approved<br />
<strong>the</strong> drug in 2010. More recently, Genentech’s Avastin<br />
(bevacizumab)—a blockbuster that has been on <strong>the</strong> <strong>market</strong><br />
since 2004 (and approved in 2008 for breast cancer)—had<br />
its approval for breast cancer revoked because later studies<br />
showed insufficient life extension or improvement in quality<br />
of life. (The drug is still approved for o<strong>the</strong>r cancers, and is<br />
being used off-label by some breast cancer patients).<br />
Fig. 2. IMS <strong>Health</strong>’s count of top global <strong>oncology</strong><br />
manufacturers (left) and top products (right)<br />
Deciding which indication or indications to pursue (when<br />
<strong>the</strong> agent has shown preliminary effectiveness in several<br />
tumor types and hematologic disorders) is anything but<br />
straightforward. When a promising <strong>oncology</strong> compound<br />
has several potential target indications in different cancers or<br />
tumor types, it’s up to drug companies to decide how broad a<br />
<strong>the</strong>rapeutic footprint to pursue, and identify which indications<br />
may represent <strong>the</strong> most realistic or lucrative opportunity — a<br />
niche vs. more comprehensive <strong>market</strong> opportunity. The<br />
decision involves tradeoffs between clinical and commercial<br />
considerations. “It’s common to see a fierce internal struggle<br />
within a given drug company, as some stakeholders want to<br />
follow <strong>the</strong> science and let clinical drivers guide <strong>the</strong> decision,<br />
while o<strong>the</strong>rs would prefer to let commercial issues (related<br />
to <strong>market</strong> size, potential ease of regulatory approval, and<br />
potential for high pricing) guide <strong>the</strong> process,” says Wen Shi,<br />
practice executive for Campbell Alliance (New York, NY), <strong>the</strong><br />
consulting arm of inVentiv <strong>Health</strong>.<br />
While large patient populations have traditionally been<br />
<strong>the</strong> primary focus in many disease categories, focusing on<br />
Biomarkers create treatment opportunities, headaches<br />
There may be no better example of how advancing medical science creates opportunities<br />
(and saves lives)—while generating operational and economic problems for healthcare<br />
systems and drug manufacturers—than biomarkers. It is now well-established that some<br />
oncolytics are “targeted” for specific genetic profiles, and some tests give signals of <strong>the</strong><br />
advent or progression of cancer, thus guiding treatment options.<br />
Today, some 25% of Phase III assets in <strong>oncology</strong> have associated biomarker programs;<br />
and <strong>the</strong> majority of leading <strong>oncology</strong> companies, including Roche, Novartis, Bristol-Myers<br />
Squibb, Merck, Pfizer, Eli Lilly and GlaxoSmithKline, have stated <strong>the</strong>ir intent to develop<br />
biomarkers for most or all of <strong>the</strong>ir drugs in clinical development, says Wen Shi of Campbell<br />
Alliance. “Having a strong biomarker program has increasingly become a cost of doing<br />
business ra<strong>the</strong>r than <strong>the</strong> true differentiator it once was.”<br />
The drawbacks to biomarker use are <strong>the</strong> <strong>complex</strong>ity and uncertainty for payers, and <strong>the</strong><br />
potential reduction in <strong>market</strong> for a drug. Many healthcare plans are struggling to decide<br />
which biomarkers to test, which genetic tests to include in <strong>the</strong>ir coverage decisions, and what<br />
levels of coverage to provide. “Payers are acutely aware of <strong>the</strong> proliferation of genetic test<br />
options, but <strong>the</strong>y are scrambling to analyze <strong>the</strong> scientific data and complete <strong>the</strong> necessary<br />
patient care and financial impact analyses,” says Neely of Xcenda.<br />
For manufacturers, a diagnostic test can reduce its potential use dramatically. In 2004,<br />
Erbitux (cetuximab; Eli Lilly/BMS/Merck Serono) was approved for colorectal cancer<br />
(CRC) patients that expressed <strong>the</strong> epidermal growth factor receptor (EGFR)—which<br />
represent <strong>the</strong> vast majority of CRC patients. However, a retrospective analysis published in<br />
2007 indicated a lack of response to Erbitux in CRC patients with ano<strong>the</strong>r biomarker—<strong>the</strong><br />
KRAS mutation. As a result, FDA added a notation on <strong>the</strong> label indications for both Erbitux<br />
and Vectibix (panitumumab; Amgen/Takeda; a similar drug to Erbitux) to specifically<br />
recommend against <strong>the</strong> use of <strong>the</strong>se drugs on KRAS-mutant CRC patients. “With FDA’s<br />
changes, <strong>the</strong> <strong>market</strong> for <strong>the</strong>se two drugs was cut in half overnight; <strong>the</strong>se companies<br />
cannot just double <strong>the</strong>ir prices to make up for that,” says Shi of Campbell Alliance. “In<br />
addition, payers now require <strong>the</strong> KRAS test to be conducted before <strong>the</strong>se two drugs can be<br />
considered.”<br />
In <strong>the</strong> final analysis, however, payers will benefit from targeted <strong>the</strong>rapies that avoid<br />
patients for whom <strong>the</strong> drugs will do no good; and manufacturers can get products to <strong>market</strong><br />
that might o<strong>the</strong>rwise remain in <strong>the</strong> laboratory because clinical results in undifferentiated<br />
populations were too marginal to support additional development.<br />
Visit our new website at www.PharmaceuticalCommerce.com May | June 2012
Brand Marketing &<br />
Communications<br />
areas of unmet medical need in <strong>oncology</strong><br />
may offer better prospects in terms of rapid<br />
regulatory approval and more attractive<br />
payer reimbursement. Too often, aiming for<br />
a larger population can backfire in <strong>oncology</strong>,<br />
because <strong>the</strong> efficacy data becomes diluted<br />
when highly specific <strong>the</strong>rapies fail to work<br />
on a certain percentage of patients, and this<br />
can undermine both insurer and prescriber<br />
support. In <strong>the</strong>se cases, <strong>the</strong> ability to segment<br />
and focus <strong>the</strong> patient population—often<br />
through <strong>the</strong> use of biomarkers—for <strong>the</strong> sake<br />
of a stronger value proposition can make all<br />
<strong>the</strong> difference.<br />
“Population size can certainly influence<br />
<strong>the</strong> decision, with larger populations offering<br />
a potentially more lucrative opportunity,”<br />
says Stephanie Hawthorne, PhD, a director<br />
at <strong>Kantar</strong> <strong>Health</strong>. “However, if multiple<br />
agents with <strong>the</strong> same mechanism are being<br />
developed in one indication, it may prove<br />
difficult to differentiate your agent, especially<br />
if you are not first-to-<strong>market</strong>; this could<br />
drive development toward a less competitive<br />
alternative indication.”<br />
“By targeting an area with few or no<br />
effective treatments, a manufacturer can<br />
potentially accelerate <strong>the</strong> time between<br />
discovery, approval and commercialization,<br />
and accelerate <strong>the</strong> uptake in <strong>the</strong> targeted<br />
area,” adds Adrian Barfield, GM of <strong>the</strong><br />
pharmaceutical and biotech group of<br />
Cardinal <strong>Health</strong> Specialty Solutions (Dublin,<br />
OH).<br />
“An increasingly common approach is<br />
to obtain initial FDA approval in an orphan<br />
indication and <strong>the</strong>n access <strong>the</strong> larger<br />
populations via compendia-supported<br />
off-label utilization in larger tumor types,”<br />
says Doug Neely, CMPE, MHA, senior<br />
director for Xcenda, a business unit of<br />
AmerisourceBergen Consulting Services<br />
(Valley Forge, PA). However, “that can set<br />
up unique pricing challenges,” adds Loreen<br />
Brown, MSW, an SVP at Xcenda.<br />
“The indication of first launch will set<br />
<strong>the</strong> price of <strong>the</strong> drug for that tumor type,<br />
but it could have repercussions for later<br />
line extensions if a significantly higher dose<br />
(which may prove too expensive) or a lower<br />
dose (which may undervalue <strong>the</strong> product)<br />
is approved,” adds Hawthorne of <strong>Kantar</strong><br />
<strong>Health</strong>. “Meanwhile, in some indications,<br />
particularly those with no <strong>the</strong>rapeutic options<br />
and high unmet need, <strong>oncology</strong> products<br />
may obtain accelerated regulatory approval<br />
based on limited data, such as a single arm<br />
Phase II trial or interim data for a surrogate<br />
endpoint—with <strong>the</strong> goal of getting an active<br />
agent to <strong>market</strong> more quickly.”<br />
“Paradoxically, while regulators may<br />
be willing to fast-track certain anti-cancer<br />
agents—as much as 37% of all oncolytics,<br />
according to Xcenda—<strong>the</strong>y are also<br />
increasingly calling for more proof of<br />
definitive overall survival gains during <strong>the</strong><br />
regulatory review, especially when o<strong>the</strong>r<br />
treatment options already exist for that<br />
indication,” says <strong>Kantar</strong> <strong>Health</strong>’s Warner.<br />
Market access<br />
It’s rare to see a cancer drug in eveningtelevision<br />
DTC ads, for <strong>the</strong> simple reason<br />
that <strong>the</strong>ir utilization is a <strong>complex</strong> interplay of<br />
cancer types, biomarkers, genetic mutations<br />
and step <strong>the</strong>rapies. At <strong>the</strong> same time,<br />
oncologists are renowned for being <strong>the</strong> most<br />
demanding of relevant clinical data in making<br />
<strong>the</strong>rapy decisions (Pharmaceutical Commerce<br />
Mar/Apr 2011, p. 1). For <strong>the</strong>se reasons,<br />
<strong>oncology</strong> product managers are in continuous<br />
contact with oncologists <strong>the</strong>mselves, and that<br />
leads to <strong>the</strong> group purchasing organizations<br />
and practice-management companies that<br />
serve oncologists and hematologists.<br />
Traditionally, <strong>the</strong> “buy-and-bill” model<br />
has dominated <strong>the</strong> <strong>oncology</strong> drug sales<br />
channel; and today, more than 95% of<br />
distribution in <strong>oncology</strong> flows through a<br />
handful of GPOs, including Oncology Supply<br />
(part of AmerisourceBergen Specialty Group,<br />
and <strong>the</strong> largest dedicated <strong>oncology</strong> GPO),<br />
VitalSource GPO (owned by Cardinal <strong>Health</strong><br />
Specialty Solutions), McKesson and o<strong>the</strong>rs.<br />
“GPOs facilitate better pricing through<br />
aggregated purchasing from manufacturers as<br />
<strong>the</strong>y are able to negotiate with manufacturers<br />
to create volume- and <strong>market</strong> share-based<br />
incentive contracts,” says Bruce Feinberg,<br />
chief medical officer, <strong>oncology</strong> for Cardinal<br />
<strong>Health</strong> Specialty Solutions. Related practicemanagement<br />
businesses at <strong>the</strong>se companies<br />
include P4 (now owned by Cardinal) and<br />
ION Solutions (AmerisourceBergen Specialty<br />
Group).<br />
The <strong>oncology</strong> GPO not only aggregates<br />
purchase demand to lower prices for its<br />
member physicians, but it can also be an<br />
aggregator of data about <strong>the</strong> behavior of those<br />
same member physicians, says Feinberg. For<br />
instance, valuable pharmacy data, outcomes<br />
data, and information about side effects and<br />
adverse reactions can be aggregated at <strong>the</strong><br />
practice level and linked with patient medical<br />
information, adds Michael (Mick) Koerner,<br />
RPh, a senior director at ION Solutions.<br />
Feinberg says that analyzing patterns<br />
of care and purchasing trends can help to<br />
identify rates of new <strong>the</strong>rapy adoption, areas<br />
of significant variance in treatment and more;<br />
and such data can <strong>the</strong>n drive clinical trial<br />
development and comparative-effectiveness<br />
research. “In our view, <strong>the</strong> GPO of <strong>the</strong> future<br />
will be much more like a trusted advisor and<br />
consultant that can provide competitively<br />
priced medications.”<br />
Practice-management groups are centrally<br />
involved in one of <strong>the</strong> o<strong>the</strong>r distinct trends<br />
in <strong>oncology</strong> <strong>market</strong> access: clinical pathways.<br />
Driven mostly by payer pressure, <strong>the</strong><br />
practice networks work with oncologists to<br />
develop preferred treatment progressions<br />
based on <strong>the</strong> type of cancer and <strong>the</strong> patient’s<br />
response. These standardized protocols<br />
are published and regularly updated by <strong>the</strong><br />
National Comprehensive Cancer Network<br />
(NCCN), ASCO, Innovent Oncology and<br />
o<strong>the</strong>r stakeholder groups. Today, some<br />
payers offer incentives or more advantageous<br />
reimbursement strategies when <strong>the</strong> course<br />
of treatment follows an approved treatment<br />
pathway. “Similar to getting a drug on<br />
formulary, manufacturers need to work<br />
collaboratively with providers and payers<br />
to ensure that <strong>the</strong>ir product is positioned<br />
appropriately/favorably within <strong>the</strong> relevant<br />
pathways,” says Brown of Xcenda.<br />
In addition to dispensing <strong>oncology</strong> drugs,<br />
<strong>oncology</strong>-oriented specialty pharmacies<br />
May | June 2012 Visit our new website at www.PharmaceuticalCommerce.com
Brand Marketing &<br />
Communications<br />
<strong>Navigating</strong> <strong>the</strong> <strong>tense</strong>, <strong>complex</strong> <strong>oncology</strong> <strong>market</strong><br />
and GPOs provide an increasingly broad range of services,<br />
including advanced clinical support to patients, support with<br />
side effect management, improved compliance support, and<br />
administrative assistance in gaining insurance coverage or<br />
identifying additional financial assistance for patients (such<br />
as drug-company-sponsored patient assistance programs for<br />
those who need it).<br />
However, under <strong>the</strong> buy-and-bill model, “oncologists face<br />
increased risk that <strong>the</strong>y will have administered a high-cost<br />
<strong>the</strong>rapy only to find that <strong>the</strong>ir charge is denied payment or<br />
payment is significantly delayed, and a growing number of<br />
patients are unable to afford <strong>the</strong>ir portion of <strong>the</strong> bill,” says<br />
Warner of <strong>Kantar</strong> <strong>Health</strong>.<br />
In 2005, CMS implemented significant changes in <strong>the</strong><br />
way it pays oncologists for physician-administered drugs<br />
and related services—changes that continue to influence<br />
<strong>the</strong> landscape in <strong>oncology</strong> today. At that time, Medicare<br />
changed its policies to set payments for covered drugs at <strong>the</strong><br />
Average Sales Price (ASP) rate plus 6%, which is based on<br />
actual transaction prices and, basically, less than <strong>the</strong> previous<br />
setpoint, Average Wholesale Price (AWP). “Prior to 2005, it<br />
was not uncommon for oncologists to make more than 50%<br />
markup on a class of drugs that was already growing at 25%<br />
per year, so when patients were not able to pay <strong>the</strong>ir co-pays,<br />
it didn’t really matter,” says OncoMed’s Zweigenhaft. “The<br />
earlier CMS rules, which allowed for huge margins, set up a<br />
whole set of perverse economics, ra<strong>the</strong>r than economics based<br />
on sound outcomes data,” says Zweigenhaft. “Cancer is still<br />
not managed that way—but it should be.”<br />
With current reimbursement rates dramatically reduced,<br />
many physicians would be forced to operate at a loss if <strong>the</strong>y<br />
were unable to collect <strong>the</strong> patients’ co-payment. “In certain<br />
situations, it is becoming increasingly common for patients<br />
to be referred to <strong>the</strong> hospital out-patient setting, so that <strong>the</strong>ir<br />
anti-cancer medications can be billed through <strong>the</strong> oftenmore-generous<br />
medication benefit, ra<strong>the</strong>r than <strong>the</strong> pharmacy<br />
benefit, portion of <strong>the</strong>ir coverage, due to <strong>the</strong>se financial<br />
dynamics,” says Warner of <strong>Kantar</strong> <strong>Health</strong>.<br />
In an effort to eliminate some of <strong>the</strong> financial incentives<br />
tied to drug administration in <strong>the</strong> buy-and-bill model, some<br />
payer plans now require that oncologists order <strong>the</strong>ir infused<br />
drugs on a patient-by-patient basis from a specific specialty<br />
pharmacy. The pharmacy delivers <strong>the</strong> drugs to <strong>the</strong> practice<br />
at <strong>the</strong> time <strong>the</strong> patient has <strong>the</strong>ir infusion visit. “In this newer<br />
model, <strong>the</strong> payer pays <strong>the</strong> specialty pharmacy directly for <strong>the</strong><br />
drugs and <strong>the</strong> physician only bills <strong>the</strong> drug administration<br />
fee,” explains Warner of <strong>Kantar</strong> <strong>Health</strong>. “While this eliminates<br />
<strong>the</strong> profit incentive, this model can result in significant<br />
drug waste if <strong>the</strong> patient is not able to receive <strong>the</strong>ir intended<br />
chemo<strong>the</strong>rapy because <strong>the</strong> drugs cannot be returned or used<br />
for a different patient.”<br />
Not surprisingly, it is also “very unpopular” with<br />
oncologists, Warner adds. “The greatest advantage of <strong>the</strong><br />
traditional buy-and-bill model is that a large inventory is<br />
maintained at <strong>the</strong> oncologists’ practice setting, so physicians<br />
are able to tailor <strong>the</strong> exact dose and combination of drugs to<br />
patients as <strong>the</strong>y arrive for <strong>the</strong>ir visit.”<br />
Altos Solutions, a Los Altos, CA-based IT and <strong>oncology</strong><br />
consulting company, performs a National Oncology Practice<br />
Benchmark annually. In its 2011 Benchmark (published<br />
in <strong>the</strong> November issue of J. of Oncology Practice, an ASCO<br />
publication), it reports that drug revenue as a percentage<br />
of total practice revenue has declined from 85% in 2005 to<br />
about 66% in 2010, and that <strong>the</strong> drug margin (i.e., profit) has<br />
declined from 22% to 9% (as a percentage of total revenue)<br />
over <strong>the</strong> same period. Although drug margin is clearly not <strong>the</strong><br />
only source of revenue for community <strong>oncology</strong> practices, it<br />
has been an important one. In a previous report (published<br />
in <strong>the</strong> September J. of Oncology Practice), <strong>the</strong> Altos authors<br />
conclude:<br />
“As <strong>the</strong> economic model for community practice<br />
transitions from one that relied on <strong>the</strong> profit margin from<br />
drugs, <strong>the</strong> question becomes what is <strong>the</strong> next economic<br />
model? … Clearly, appropriate use of [new diagnostics and<br />
sophisticated tumor typing] will demand more physician<br />
time, not less. … Although <strong>the</strong> benefits of new diagnostic<br />
and treatment tools are desired by everyone involved in<br />
cancer care—patients, providers and payers—<strong>the</strong>y cannot<br />
be delivered in <strong>the</strong> existing community <strong>oncology</strong> economic<br />
business model.”<br />
Off-label drug use in <strong>oncology</strong><br />
Given <strong>the</strong> limited number of <strong>oncology</strong> agents available and<br />
<strong>the</strong> many types of cancers that are encountered, <strong>the</strong> off-label<br />
use of medications in cancer treatment has been widespread<br />
for decades. Today, estimates show that more than half of all<br />
uses of cancer-treating drugs are prescribed off-label, notes<br />
Brown of Xcenda.<br />
While having more options to choose from may offer an<br />
ongoing ray of hope for oncologists and patients who are<br />
running out of treatment options, “<strong>the</strong> quality of clinical<br />
evidence to support off-label use for a given patient may be<br />
weak, <strong>the</strong>reby exposing patients to ineffective and potentially<br />
toxic <strong>the</strong>rapy, and creating unnecessary expenses for payers,”<br />
says Harish Dave, MD, MBA, VP of <strong>the</strong> Oncology Therapeutic<br />
Delivery Unit of Quintiles (Rockville, MD). As a result, to<br />
curtail healthcare costs, payers are actively working to curtail<br />
off-label prescribing in <strong>oncology</strong>.<br />
Today, <strong>the</strong> majority of cancer patients—roughly 60%—are<br />
on Medicare. When it comes to Medicare, coverage of offlabel<br />
drug use in <strong>oncology</strong> is limited to those agents that are<br />
listed in at least one of four accepted compendia:<br />
• American Hospital Formulary Service Drug Information<br />
• Gold Standard Inc. Clinical Pharmacology Compendium<br />
• National Comprehensive Cancer Network (NCCN)<br />
Drugs and Biologics Compendium<br />
• Thomson Micromedex DrugDex Compendium<br />
“Today, most private payers follow Medicare coverage<br />
policy when setting <strong>the</strong>ir own criteria. Thus, payers are<br />
increasingly refusing to pay for treatments off-label, unless<br />
<strong>the</strong>y have a compendia listing or sufficient evidence to support<br />
<strong>the</strong>ir clinical value,” says Warner of <strong>Kantar</strong> <strong>Health</strong>. “For this<br />
reason, it’s in <strong>the</strong> drug company’s best interest to actively<br />
pursue a solid compendia strategy.”<br />
Oral meds and self-injectables<br />
Yet ano<strong>the</strong>r <strong>complex</strong>ity of oncolytics is that <strong>the</strong> form of<br />
drug delivery can affect its affordability, patient acceptance<br />
and, indirectly, its efficacy. Traditionally, chemo<strong>the</strong>rapy has<br />
been administered via intravenous infusion, but over <strong>the</strong> past<br />
decade, <strong>the</strong>re has been a surge of newer oral and self-injected<br />
agents. Some are traditional chemo<strong>the</strong>rapies, such as Xeloda<br />
(capecitabine), while o<strong>the</strong>rs are targeted <strong>the</strong>rapies, such as<br />
Gleevec (imatinib) and Sutent (sunitinib). Today, of <strong>the</strong> 125<br />
drugs in Phase III clinical development for cancer, 38 are oral<br />
medications, according to Medco.<br />
For drug developers, <strong>the</strong> decision about whe<strong>the</strong>r<br />
to pursue <strong>the</strong> development of a compound as an oral or<br />
injectable <strong>the</strong>rapy will be dictated by clinical and commercial<br />
considerations. If <strong>the</strong> drug’s side effect profile supports selfadministration,<br />
<strong>the</strong>re are some key benefits for <strong>the</strong> right<br />
patients, says Koerner of ION Solutions. These include <strong>the</strong><br />
freedom for patients to take <strong>the</strong>ir medications at home (saving<br />
time and travel), and in some instances, increased insurance<br />
coverage, as <strong>the</strong> pharmacy benefit may be preferential to <strong>the</strong><br />
medical benefit in terms of patient co-pays and administrative<br />
requirements for oral <strong>oncology</strong> medications.<br />
Meanwhile, <strong>the</strong> pursuit of self-injectable <strong>oncology</strong> meds<br />
is also garnering attention. For instance, Roche is currently<br />
developing subcutaneous reformulations of Herceptin and<br />
Rituxan (rituximab). The potential benefit for <strong>the</strong> patient<br />
would be shorter medical visits, as <strong>the</strong> subcutaneous injection<br />
(ra<strong>the</strong>r than <strong>the</strong> IV infusion) can be given in a few minutes<br />
ra<strong>the</strong>r than hours, or possibly at-home administration. “This<br />
is particularly attractive for <strong>the</strong>se two agents when <strong>the</strong>y are<br />
used in <strong>the</strong> adjuvant/maintenance settings with treatment<br />
lasting for more than a year,” says Kate Keeping, principal<br />
analyst at Decision Resources, a <strong>market</strong> research company.<br />
“From a commercial perspective, this also offers Roche <strong>the</strong><br />
opportunity to offset <strong>the</strong> imminent threat of biosimilar<br />
competition to <strong>the</strong> intravenous formulations of <strong>the</strong>se brands.”<br />
A subcutaneous formulation of Velcade (bortezomib;<br />
Millennium Pharmaceuticals) for multiple myeloma was<br />
recently launched, which not only offers more convenient<br />
delivery, but also reduced neurotoxicity, she says.<br />
There are drawbacks to self-administration: <strong>the</strong> risk of<br />
adherence and compliance issues, and failure to use <strong>the</strong><br />
<strong>the</strong>rapy as prescribed (in particular, missing doses) that can<br />
impact outcomes and result in premature emergence of<br />
drug resistance. And at-home administration reduces patient<br />
interaction with both physicians and nurses, which reduces<br />
opportunities for regular monitoring. US physicians, who<br />
receive a fee for administering intravenous drugs, may also<br />
resist <strong>the</strong> loss of that revenue stream.<br />
National studies confirm that overall noncompliance to<br />
oral drugs across all disease categories can run as high as 50%<br />
to 70%. “This presents a challenge in any disease state but can<br />
have a significant impact when it comes to chemo<strong>the</strong>rapy,”<br />
says Koerner of ION Solutions. “In some instances, patients<br />
may have side effects or cost pressures, and decide not to<br />
take <strong>the</strong> drug or take <strong>the</strong> drug less frequently,” says Brown of<br />
Xcenda. “In o<strong>the</strong>rs, <strong>the</strong> physician may opt to dose-reduce <strong>the</strong><br />
patient to manage through certain side effects, but <strong>the</strong> patient<br />
may want to take <strong>the</strong> full dose to ensure <strong>the</strong>y are getting <strong>the</strong><br />
maximum benefit of <strong>the</strong> drug.” With many of <strong>the</strong>se drugs<br />
costing as much as $7,500 a month, says Quigley of IMS,<br />
it serves nobody’s interest to have <strong>the</strong>m be administered<br />
incorrectly or not at all.”<br />
As for distribution, oral <strong>oncology</strong> <strong>the</strong>rapies often fall<br />
outside <strong>the</strong> traditional buy-and-bill model. In this realm,<br />
“Specialty pharmacy providers are taking on a much greater<br />
role in patient management, as office-based clinicians turn<br />
over <strong>the</strong> ongoing management for self-administered products<br />
to <strong>the</strong>m,” says Doug Neely of Xcenda. “Payers are much more<br />
comfortable managing drugs through traditional pharmacy<br />
benefit management (PBM) systems.”<br />
“With <strong>the</strong> growing availability of oral options for <strong>oncology</strong><br />
treatment, some larger <strong>oncology</strong> practices have opened<br />
dispensing pharmacies, so <strong>the</strong>y can also dispense oral cancer<br />
drugs to patients,” says Warner of <strong>Kantar</strong> <strong>Health</strong>. However,<br />
this approach can bring significant business challenges<br />
for <strong>oncology</strong> practices, so it tends to be seen only in larger<br />
<strong>oncology</strong> practices. It is thought that less than 10% of practices<br />
currently dispense oral <strong>oncology</strong> drugs directly. “In <strong>oncology</strong>,<br />
payers in turn will continue to scrutinize <strong>the</strong> incremental value<br />
of new <strong>the</strong>rapies, while experimenting with new approaches<br />
to manage overall costs, while maintaining access to quality<br />
cancer care,” she concludes. “Manufacturers need to evolve<br />
<strong>the</strong>ir clinical development, <strong>market</strong>ing and sales strategies in<br />
order to remain competitive in this <strong>complex</strong> arena.”<br />
Visit our new website at www.PharmaceuticalCommerce.com May | June 2012