Sample Assessment Schedule 2012
Sample Assessment Schedule 2012
Sample Assessment Schedule 2012
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
NCEA Level 2 Chemistry 91165 (2.5) — page 1 of 6<br />
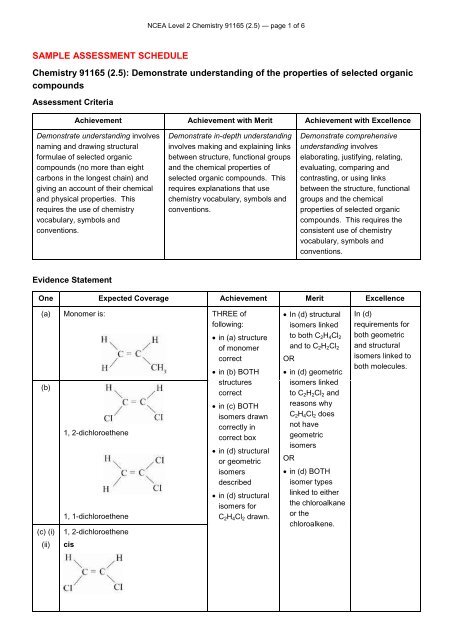
SAMPLE ASSESSMENT SCHEDULE<br />
Chemistry 91165 (2.5): Demonstrate understanding of the properties of selected organic<br />
compounds<br />
<strong>Assessment</strong> Criteria<br />
Achievement Achievement with Merit Achievement with Excellence<br />
Demonstrate understanding involves<br />
naming and drawing structural<br />
formulae of selected organic<br />
compounds (no more than eight<br />
carbons in the longest chain) and<br />
giving an account of their chemical<br />
and physical properties. This<br />
requires the use of chemistry<br />
vocabulary, symbols and<br />
conventions.<br />
Demonstrate in-depth understanding<br />
involves making and explaining links<br />
between structure, functional groups<br />
and the chemical properties of<br />
selected organic compounds. This<br />
requires explanations that use<br />
chemistry vocabulary, symbols and<br />
conventions.<br />
Demonstrate comprehensive<br />
understanding involves<br />
elaborating, justifying, relating,<br />
evaluating, comparing and<br />
contrasting, or using links<br />
between the structure, functional<br />
groups and the chemical<br />
properties of selected organic<br />
compounds. This requires the<br />
consistent use of chemistry<br />
vocabulary, symbols and<br />
conventions.<br />
Evidence Statement<br />
One Expected Coverage Achievement Merit Excellence<br />
(a) Monomer is: THREE of<br />
following:<br />
(b)<br />
(c) (i)<br />
(ii)<br />
1, 2-dichloroethene<br />
1, 1-dichloroethene<br />
1, 2-dichloroethene<br />
cis<br />
in (a) structure<br />
of monomer<br />
correct<br />
in (b) BOTH<br />
structures<br />
correct<br />
in (c) BOTH<br />
isomers drawn<br />
correctly in<br />
correct box<br />
in (d) structural<br />
or geometric<br />
isomers<br />
described<br />
in (d) structural<br />
isomers for<br />
C 2 H 4 Cl 2 drawn.<br />
In (d) structural<br />
isomers linked<br />
to both C 2 H 4 Cl 2<br />
and to C 2 H 2 Cl 2<br />
OR<br />
in (d) geometric<br />
isomers linked<br />
to C 2 H 2 Cl 2 and<br />
reasons why<br />
C 2 H 4 Cl 2 does<br />
not have<br />
geometric<br />
isomers<br />
OR<br />
in (d) BOTH<br />
isomer types<br />
linked to either<br />
the chloroalkane<br />
or the<br />
chloroalkene.<br />
In (d)<br />
requirements for<br />
both geometric<br />
and structural<br />
isomers linked to<br />
both molecules.
NCEA Level 2 Chemistry 91165 (2.5) — page 2 of 6<br />
trans<br />
(d)<br />
Structural isomers are compounds with<br />
the same molecular formula (they have<br />
the same type and number of atoms) but<br />
different structural formulae (structures).<br />
C 2 H 4 Cl 2 has two structural isomers, one<br />
has a condensed structural formula<br />
CH 2 Cl–CH 2 Cl and the other CH 3 –CHCl 2<br />
C 2 H 2 Cl 2 also has structural isomers, one<br />
has a condensed structural formula<br />
CH 2 Cl–CHCl and the other CH 2 –CCl 2<br />
Geometric isomers are compounds with<br />
the same molecular formula, the same<br />
(condensed) structural formula but<br />
different arrangement of atoms. It occurs<br />
in molecules that have double bonds<br />
because the rotation of the atoms about<br />
the axis of the carbon to carbon double<br />
bond is restricted. They must also have<br />
two different groups attached to each end<br />
of the double bond.<br />
C 2 H 4 Cl 2 doesn’t have a double bond; it<br />
has a single bond between the carbon<br />
atoms. Rotation of the atoms about this<br />
bond can occur freely so C 2 H 4 Cl 2 cannot<br />
form geometric isomers.<br />
C 2 H 2 Cl 2 has a double bond and each<br />
carbon involved has two different groups<br />
attached to them so C 2 H 2 Cl 2 can form<br />
geometric isomers.
NCEA Level 2 Chemistry 91165 (2.5) — page 3 of 6<br />
NØ<br />
N1<br />
N2<br />
A3<br />
A4<br />
M5<br />
M6<br />
E7<br />
E8<br />
No response or does not address the question.<br />
Provides some writing but does not fulfil any statement from the Achievement criteria column.<br />
Any ONE statement from the Achievement criteria column.<br />
Any TWO statements from the Achievement criteria column.<br />
Any THREE statements from the Achievement criteria column.<br />
Any ONE statements from the Merit criteria column.<br />
Gives BOTH statements from the Merit criteria column.<br />
Only minor error – omission or additional information – from the Excellence criteria column.<br />
All the evidence from the Excellence criteria column.<br />
Two Expected Coverage Achievement Merit Excellence<br />
(a) See Appendix One. THREE of the<br />
(b) Water<br />
following:<br />
Add water to the five solutions. Three<br />
solutions will dissolve in water<br />
(ethanol, aminoethane, ethanoic<br />
acid), two will not (hexane, hex-1-<br />
ene).<br />
Litmus<br />
Use the solutions formed by<br />
dissolving in water. Add both red and<br />
blue litmus paper to the solutions in<br />
water.<br />
One will not change the colour of the<br />
litmus paper, this is ethanol.<br />
One will turn blue litmus red, this is<br />
ethanoic acid.<br />
One will turn red litmus blue, this is<br />
aminoethane.<br />
Test the solutions which did not<br />
dissolve in water by reacting fresh<br />
solutions with bromine water. If the<br />
solution turns from brown to<br />
colourless the solution is hex-1-ene.<br />
Hexane will not react with the<br />
bromine water.<br />
Equations with water -<br />
CH 3 COOH + H 2 O CH 3 COO – +<br />
H 3 O +<br />
in (a) THREE<br />
names or<br />
structural<br />
formulae correct<br />
in (a) THREE<br />
homologous<br />
series identified<br />
or functional<br />
groups correct<br />
in (b) reaction<br />
with two of water,<br />
litmus or bromine<br />
water described<br />
with observations<br />
for one solution<br />
OR any two<br />
observations<br />
in (b) reaction<br />
with one of<br />
water, litmus or<br />
bromine water<br />
described with<br />
observations for<br />
three (different)<br />
solutions.<br />
OR any one<br />
solution correctly<br />
identified<br />
In (b) THREE<br />
solutions identified<br />
with all tests<br />
required to<br />
positively identify.<br />
In (b) writes a valid<br />
method that<br />
distinguishes<br />
between the five<br />
solutions. At least<br />
two equations are<br />
included.
NCEA Level 2 Chemistry 91165 (2.5) — page 4 of 6<br />
CH 3 CH 2 NH 2 + H 2 O CH 3 CH 2 NH 3<br />
+<br />
+<br />
OH –<br />
Equation with bromine water –<br />
CH 3 – CH 2 – CH 2 – CH 2 – CH=CH 2 +<br />
Br 2 CH 3 – CH 2 – CH 2 – CH 2 – CHBr–<br />
CH 2 Br<br />
NØ<br />
N1<br />
N2<br />
A3<br />
A4<br />
M5<br />
M6<br />
E7<br />
E8<br />
No response or does not address the question.<br />
Provides some writing but does not fulfil any statement from the Achievement criteria column.<br />
Any ONE statement from the Achievement criteria column.<br />
Any TWO statements from the Achievement criteria column.<br />
Any THREE statements from the Achievement criteria column.<br />
TWO solutions identified with all tests to positively identify.<br />
Gives BOTH statements from the Merit criteria column.<br />
Only minor error – omission or additional information – from the Excellence criteria column.<br />
All the evidence from the Excellence criteria column.<br />
Three Expected Coverage Achievement Merit Excellence<br />
(a)<br />
(b) (i)<br />
Compound A is CH 3 –CH 2 –CH 2 –OH<br />
Compound B is CH 3 –CH 2 –CH 2 –NH 2<br />
THREE of the<br />
following:<br />
in (a) compound<br />
(ii) Reactant P is HCI<br />
A correct<br />
Reactant Q is concentrated H 2 SO 4<br />
in (b) TWO out<br />
Reactant R is HCl<br />
of FOUR<br />
(c) (i)<br />
(ii)<br />
(1) substitution.<br />
(2) substitution.<br />
(3) elimination.<br />
(4) addition.<br />
compounds of<br />
reactants<br />
correct<br />
in (c)(i) TWO<br />
reaction types<br />
described but<br />
not linked to the<br />
atoms involved<br />
Molecules that undergo substitution<br />
reactions have carbon to carbon single<br />
bonds and form molecules with carbon<br />
to carbon single bonds. In a substitution<br />
reaction an atom or group of atoms is<br />
replaced by another atom or group of<br />
atoms.<br />
Molecules that undergo elimination<br />
reactions have carbon to carbon single<br />
bonds and form molecules with carbon<br />
to carbon double bonds. In an<br />
elimination reaction two atoms or small<br />
groups are removed from a molecule<br />
forming a carbon to carbon to double<br />
bond.<br />
Molecules that undergo addition<br />
in (c) (ii) ONE<br />
reaction type<br />
described.<br />
In (c) THREE<br />
reactions correct<br />
in terms of<br />
reactant, product<br />
and reaction type<br />
which is linked to<br />
a reason for the<br />
reaction type.<br />
In (c) THREE<br />
reactions correct<br />
in terms of both<br />
reactant, product<br />
and reaction type<br />
AND the<br />
justification of<br />
reaction type for<br />
that reaction.
NCEA Level 2 Chemistry 91165 (2.5) — page 5 of 6<br />
reactions have carbon to carbon double<br />
bonds and form molecules with carbon<br />
to carbon single bonds. In an addition<br />
reaction the reaction involves breaking<br />
a double bond between the carbon<br />
atoms and forming a single bond in its<br />
place as well as forming two new single<br />
bonds.<br />
Reactions (1) and (2) are both<br />
substitution reactions as the molecules<br />
have carbon to carbon single bonds. In<br />
(1) the hydroxyl group (–OH) is<br />
replaced by a chloro group (–Cl). In (2)<br />
the chloro group (–Cl) is replaced by<br />
the amine group (–NH 2 ).<br />
Reaction (3) is an elimination reaction<br />
as the molecule has carbon to carbon<br />
single bonds and a double bond is<br />
formed when it reacts. A hydrogen atom<br />
and the hydroxyl group on adjacent<br />
carbon atoms are removed forming a<br />
carbon to carbon double bond.<br />
Reaction (4) is an addition reaction as<br />
the molecule has carbon to carbon<br />
double bonds and the product has<br />
carbon to carbon single bonds. In this<br />
reaction the double bond breaks<br />
forming a single bond, a hydrogen atom<br />
attaches itself to one of the carbon<br />
atoms and a chlorine atom attaches<br />
itself to the other carbon atom.<br />
NØ<br />
N1<br />
N2<br />
A3<br />
A4<br />
M5<br />
M6<br />
E7<br />
E8<br />
No response or does not address the question.<br />
Provides some writing but does not fulfil any statement from the Achievement criteria column.<br />
Any ONE statement from the Achievement criteria column.<br />
Any TWO statements from the Achievement criteria column.<br />
Any THREE statements from the Achievement criteria column.<br />
TWO reactions correct in terms of reactant, product, reaction type, which is linked to a reason for the<br />
reaction type.<br />
THREE reactions correct in terms of reactant, product, reaction type, which is linked to a reason for the<br />
reaction type.<br />
Only minor error – omission or additional information – from the Excellence criteria column.<br />
All the evidence from the Excellence criteria column.
NCEA Level 2 Chemistry 91165 (2.5) — page 6 of 6<br />
Appendix One: Question Two (a)<br />
Organic<br />
substances<br />
Structural formula<br />
IUPAC<br />
(systematic) name<br />
Homologous<br />
series<br />
A CH 3 –CH 2 –CH 2 –NH 2<br />
1-aminopropane<br />
amine<br />
B CH 3 –OH methanol<br />
alcohol<br />
2-choropentane<br />
chloroalkane/<br />
C<br />
CH 3 –CH 2 – CH 2 –CHCl–CH 3<br />
haloalkane<br />
D<br />
CH 3 –CH 2 –COOH<br />
propanoic acid<br />
carboxylic acid<br />
3, 4- dimethylpent-2-ene alkene<br />
E