ch3-ppt-notes
ch3-ppt-notes
ch3-ppt-notes
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3.1 Measurements and Their > Using and Expressing<br />
Uncertainty<br />
Measurements<br />
A measurement is a quantity that has<br />
both a number and a unit.<br />
Measurements are fundamental to the<br />
experimental sciences. For that reason,<br />
it is important to be able to make<br />
measurements and to decide whether a<br />
measurement is correct.<br />
Slide<br />
1 of 48<br />
3.1 Measurements and Their > Using and Expressing<br />
Uncertainty<br />
Measurements<br />
In scientific notation, a<br />
given number is<br />
written as the product<br />
of two numbers: a<br />
coefficient and 10<br />
raised to a power.<br />
The number of stars in a<br />
galaxy is an example<br />
of an estimate that<br />
should be expressed<br />
in scientific notation.<br />
Slide<br />
2 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Accuracy, Precision, and Error<br />
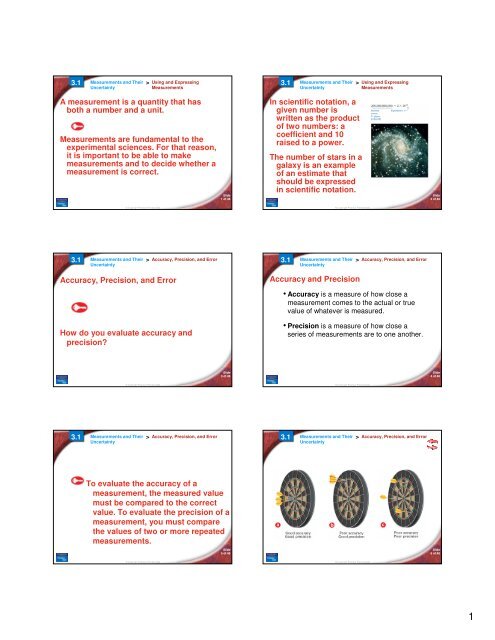
3.1 Measurements and Their > Accuracy, Precision, and Error<br />
Uncertainty<br />
Accuracy, Precision, and Error<br />
Accuracy and Precision<br />
• Accuracy is a measure of how close a<br />
measurement comes to the actual or true<br />
value of whatever is measured.<br />
How do you evaluate accuracy and<br />
precision?<br />
• Precision is a measure of how close a<br />
series of measurements are to one another.<br />
Slide<br />
3 of 48<br />
Slide<br />
4 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.1 Measurements and Their > Accuracy, Precision, and Error<br />
Uncertainty<br />
3.1 Measurements and Their > Accuracy, Precision, and Error<br />
Uncertainty<br />
To evaluate the accuracy of a<br />
measurement, the measured value<br />
must be compared to the correct<br />
value. To evaluate the precision of a<br />
measurement, you must compare<br />
the values of two or more repeated<br />
measurements.<br />
Slide<br />
5 of 48<br />
Slide<br />
6 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
1
3.1 Measurements and Their > Accuracy, Precision, and Error<br />
Uncertainty<br />
Determining Error<br />
• The accepted value is the correct value<br />
based on reliable references.<br />
3.1 Measurements and Their > Accuracy, Precision, and Error<br />
Uncertainty<br />
The percent error is the absolute value of<br />
the error divided by the accepted value,<br />
multiplied by 100%.<br />
• The experimental value is the value<br />
measured in the lab.<br />
• The difference between the experimental<br />
value and the accepted value is called the<br />
error.<br />
Slide<br />
7 of 48<br />
Slide<br />
8 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Accuracy, Precision, and Error<br />
3.1 Measurements and Their > Accuracy, Precision, and Error<br />
Uncertainty<br />
Just because a measuring device works,<br />
you cannot assume it is accurate. The<br />
scale below has not been properly<br />
zeroed, so the reading obtained for the<br />
person’s weight is inaccurate.<br />
Slide<br />
9 of 48<br />
Slide<br />
10 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Significant Figures in Measurements<br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Significant Figures in Measurements<br />
Significant Figures in Measurements<br />
Why must measurements be reported to<br />
the correct number of significant<br />
figures?<br />
Slide<br />
11 of 48<br />
Suppose you estimate a weight that is<br />
between 2.4 lb and 2.5 lb to be 2.46 lb.<br />
The first two digits (2 and 4) are known.<br />
The last digit (6) is an estimate and<br />
involves some uncertainty. All three<br />
digits convey useful information,<br />
however, and are called significant<br />
figures.<br />
The significant figures in a measurement<br />
include all of the digits that are known,<br />
plus a last digit that is estimated.<br />
Slide<br />
12 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
2
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Significant Figures in Measurements<br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Significant Figures in Measurements<br />
Measurements must always be<br />
reported to the correct number of<br />
significant figures because<br />
calculated answers often depend on<br />
the number of significant figures in<br />
the values used in the calculation.<br />
Slide<br />
13 of 48<br />
Slide<br />
14 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Measurements and Their<br />
Uncertainty<br />
><br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Significant Figures in Measurements<br />
• Cont...<br />
Slide<br />
15 of 48<br />
Slide<br />
16 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Slide<br />
17 of 48<br />
Slide<br />
18 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3
Practice Problems<br />
for Conceptual Problem 3.1<br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Significant Figures in Calculations<br />
Significant Figures in Calculations<br />
Problem Solving 3.2 Solve Problem 2<br />
with the help of an interactive guided<br />
tutorial.<br />
How does the precision of a<br />
calculated answer compare to<br />
the precision of the<br />
measurements used to obtain it?<br />
Slide<br />
19 of 48<br />
Slide<br />
20 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.1<br />
Measurements and Their<br />
Uncertainty<br />
> Significant Figures in Calculations<br />
In general, a calculated answer<br />
cannot be more precise than the<br />
least precise measurement from<br />
which it was calculated.<br />
The calculated value must be<br />
rounded to make it consistent<br />
with the measurements from<br />
which it was calculated.<br />
3.1 Measurements and Their > Significant Figures in Calculations<br />
Uncertainty<br />
Rounding<br />
To round a number, you must first decide<br />
how many significant figures your<br />
answer should have. The answer<br />
depends on the given measurements<br />
and on the mathematical process used<br />
to arrive at the answer.<br />
Slide<br />
21 of 48<br />
Slide<br />
22 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.1<br />
SAMPLE PROBLEM<br />
3.1<br />
Slide<br />
23 of 48<br />
Slide<br />
24 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
4
Practice Problems<br />
for Sample Problem 3.1<br />
3.1 Measurements and Their > Significant Figures in Calculations<br />
Uncertainty<br />
Addition and Subtraction<br />
The answer to an addition or subtraction<br />
calculation should be rounded to the<br />
same number of decimal places (not<br />
digits) as the measurement with the<br />
least number of decimal places.<br />
Problem Solving 3.3 Solve Problem 3<br />
with the help of an interactive guided<br />
tutorial.<br />
Slide<br />
25 of 48<br />
Slide<br />
26 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.2<br />
SAMPLE PROBLEM<br />
3.2<br />
Slide<br />
27 of 48<br />
Slide<br />
28 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Practice Problems for Sample Problem 3.2<br />
3.1 Measurements and Their > Significant Figures in Calculations<br />
Uncertainty<br />
Multiplication and Division<br />
• In calculations involving multiplication and<br />
division, you need to round the answer to the<br />
same number of significant figures as the<br />
measurement with the least number of<br />
significant figures.<br />
Problem Solving 3.6 Solve Problem 6<br />
with the help of an interactive guided<br />
tutorial.<br />
• The position of the decimal point has nothing<br />
to do with the rounding process when<br />
multiplying and dividing measurements.<br />
Slide<br />
29 of 48<br />
Slide<br />
30 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
5
SAMPLE PROBLEM<br />
3.3<br />
SAMPLE PROBLEM<br />
3.3<br />
Slide<br />
31 of 48<br />
Slide<br />
32 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.3<br />
Practice Problems for Sample Problem 3.3<br />
Problem Solving 3.8 Solve<br />
Problem 8 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
33 of 48<br />
Slide<br />
34 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Section Assessment<br />
Assess students’ understanding<br />
of the concepts in Section 3.1.<br />
3.1 Section Quiz<br />
1. In which of the following expressions is the<br />
number on the left NOT equal to the number<br />
on the right?<br />
Continue to:<br />
Section Quiz<br />
-or-<br />
Launch:<br />
a. 0.00456 × 10 –8 = 4.56 × 10 –11<br />
b. 454 × 10 –8 = 4.54 × 10 –6<br />
c. 842.6 × 10 4 = 8.426 × 10 6<br />
d. 0.00452 × 10 6 = 4.52 × 10 9<br />
Slide<br />
35 of 48<br />
Slide<br />
36 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
6
3.1 Section Quiz<br />
2. Which set of measurements of a 2.00-g<br />
standard is the most precise?<br />
a. 2.00 g, 2.01 g, 1.98 g<br />
b. 2.10 g, 2.00 g, 2.20 g<br />
c. 2.02 g, 2.03 g, 2.04 g<br />
d. 1.50 g, 2.00 g, 2.50 g<br />
3.1 Section Quiz<br />
3. A student reports the volume of a liquid as<br />
0.0130 L. How many significant figures are in<br />
this measurement?<br />
a. 2<br />
b. 3<br />
c. 4<br />
d. 5<br />
Slide<br />
37 of 48<br />
Slide<br />
38 of 48<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
END OF SHOW<br />
7
3.2 The International System > Measuring with SI Units<br />
of Units<br />
All measurements depend on units that serve as<br />
reference standards. The standards of<br />
measurement used in science are those of the<br />
metric system.<br />
3.2 The International System > Measuring with SI Units<br />
of Units<br />
The five SI base units commonly used<br />
by chemists are the meter, the kilogram,<br />
the kelvin, the second, and the mole.<br />
The International System of Units (abbreviated<br />
SI, after the French name, Le Système<br />
International d’Unités) is a revised version of the<br />
metric system.<br />
Slide<br />
1 of 33<br />
Slide<br />
2 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Units and Quantities<br />
What metric units are commonly used to<br />
measure length, volume, mass,<br />
temperature and energy?<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Units of Length<br />
In SI, the basic unit of length, or linear measure,<br />
is the meter (m). For very large or and very small<br />
lengths, it may be more convenient to use a unit<br />
of length that has a prefix.<br />
Slide<br />
3 of 33<br />
Slide<br />
4 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Common metric units of length include<br />
the centimeter, meter, and kilometer.<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Units of Volume<br />
The SI unit of volume is the amount of space occupied by<br />
a cube that is 1 m along each edge. This volume is the<br />
cubic meter (m) 3 . A more convenient unit of volume for<br />
everyday use is the liter, a non-SI unit.<br />
KNOW THIS:<br />
A liter (L) is the volume of a cube that is 10 centimeters<br />
(10 cm) along each edge (10 cm × 10 cm × 10 cm = 1000<br />
cm 3 = 1 L).<br />
Slide<br />
5 of 33<br />
Slide<br />
6 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
1
3.2 The International System > Units and Quantities<br />
of Units<br />
Common metric units of volume include<br />
the liter, milliliter, cubic centimeter, and<br />
microliter.<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
The volume of 20 drops of liquid from a<br />
medicine dropper is approximately 1 mL.<br />
Slide<br />
7 of 33<br />
Slide<br />
8 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
A sugar cube has a volume of 1 cm 3 . 1 mL is<br />
the same as 1 cm 3 .<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
A gallon of milk has about twice the volume of a<br />
2-L bottle of soda.<br />
Slide<br />
9 of 33<br />
Slide<br />
10 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Units of Mass<br />
The mass of an object is measured in<br />
comparison to a standard mass of 1 kilogram<br />
(kg), which is the basic SI unit of mass.<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Common metric units of mass include<br />
kilogram, gram, milligram, and<br />
microgram.<br />
A gram (g) is 1/1000 of a kilogram; the mass of 1<br />
cm 3 of water at 4°C is 1 g.<br />
Slide<br />
11 of 33<br />
Slide<br />
12 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
2
3.2 The International System > Units and Quantities<br />
of Units<br />
Weight is a force that measures the pull on a<br />
given mass by gravity.<br />
The astronaut shown on the surface of the<br />
moon weighs one sixth of what he weighs on<br />
Earth.<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Units of Temperature<br />
Temperature is a measure of<br />
how hot or cold an object is.<br />
Thermometers are used to<br />
measure temperature.<br />
Slide<br />
13 of 33<br />
Slide<br />
14 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Scientists commonly use two equivalent<br />
units of temperature, the degree Celsius<br />
and the kelvin.<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
On the Celsius scale, the freezing point of<br />
water is 0°C and the boiling point is 100°C.<br />
On the Kelvin scale, the freezing point of water<br />
is 273.15 kelvins (K), and the boiling point is<br />
373.15 K.<br />
The zero point on the Kelvin scale, 0 K, or<br />
absolute zero, is equal to −273.15 °C.<br />
Slide<br />
15 of 33<br />
Slide<br />
16 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Because one degree on the Celsius scale is<br />
equivalent to one kelvin on the Kelvin scale,<br />
converting from one temperature to another is<br />
easy. You simply add or subtract 273, as shown<br />
in the following equations.<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Conversions Between the Celsius and Kelvin<br />
Scales<br />
Slide<br />
17 of 33<br />
Slide<br />
18 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3
SAMPLE PROBLEM<br />
3.4<br />
SAMPLE PROBLEM<br />
3.4<br />
Slide<br />
19 of 33<br />
Slide<br />
20 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.4<br />
SAMPLE PROBLEM<br />
3.4<br />
Slide<br />
21 of 33<br />
Slide<br />
22 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Practice Problems<br />
for Sample Problem 3.4<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
Units of Energy<br />
Energy is the capacity to do work or to produce<br />
heat.<br />
The joule and the calorie are common<br />
units of energy.<br />
Problem Solving 3.17 Solve<br />
Problem 17 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
23 of 33<br />
Slide<br />
24 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
4
3.2 The International System > Units and Quantities<br />
of Units<br />
The joule (J) is the SI unit of energy.<br />
One calorie (cal) is the quantity of heat that<br />
raises the temperature of 1 g of pure water by<br />
1°C.<br />
3.2 The International System > Units and Quantities<br />
of Units<br />
This house is equipped with solar panels. The<br />
solar panels convert the radiant energy from the<br />
sun into electrical energy that can be used to<br />
heat water and power appliances.<br />
Slide<br />
25 of 33<br />
Slide<br />
26 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 Section Quiz.<br />
Assess students’ understanding<br />
of the concepts in Section 3.2.<br />
Continue to:<br />
Launch:<br />
-or-<br />
Section Quiz<br />
3.2 Section Quiz.<br />
1. Which of the following is not a base SI unit?<br />
a. meter<br />
b. gram<br />
c. second<br />
d. mole<br />
Slide<br />
27 of 33<br />
Slide<br />
28 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.2 Section Quiz.<br />
2. If you measured both the mass and weight of<br />
an object on Earth and on the moon, you<br />
would find that<br />
a. both the mass and the weight do not<br />
change.<br />
b. both the mass and the weight change.<br />
c. the mass remains the same, but the weight<br />
changes.<br />
d. the mass changes, but the weight remains<br />
the same.<br />
3.2 Section Quiz.<br />
3. A temperature of 30 degrees Celsius is<br />
equivalent to<br />
a. 303 K.<br />
b. 300 K.<br />
c. 243 K.<br />
d. 247 K.<br />
Slide<br />
29 of 33<br />
Slide<br />
30 of 33<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
5
END OF SHOW<br />
6
3.3 Conversion Problems > Conversion Factors<br />
A conversion factor is a ratio of equivalent<br />
measurements.<br />
The ratios 100 cm/1 m and 1 m/100 cm are<br />
examples of conversion factors.<br />
Animation 3<br />
Conversion Problems > Conversion Factors<br />
Learn how to select the proper conversion<br />
factor and how to use it.<br />
Slide<br />
1 of 43<br />
Slide<br />
2 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.3 Conversion Problems > Conversion Factors<br />
When a measurement is multiplied by<br />
a conversion factor, the numerical<br />
value is generally changed, but the<br />
actual size of the quantity measured<br />
remains the same.<br />
3.3 Conversion Problems > Conversion Factors<br />
The scale of the micrograph is in nanometers.<br />
Using the relationship 10 9 nm = 1 m, you can write<br />
the following conversion factors.<br />
Slide<br />
3 of 43<br />
Slide<br />
4 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.3 Conversion Problems > Dimensional Analysis<br />
SAMPLE PROBLEM 3.5<br />
Dimensional analysis is a way to analyze and<br />
solve problems using the units, or dimensions,<br />
of the measurements.<br />
Dimensional analysis provides you<br />
with an alternative approach to<br />
problem solving.<br />
Slide<br />
5 of 43<br />
Slide<br />
6 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
1
SAMPLE PROBLEM<br />
3.5<br />
SAMPLE PROBLEM<br />
3.5<br />
Slide<br />
7 of 43<br />
Slide<br />
8 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Practice Problems for Sample Problem 3.5<br />
SAMPLE PROBLEM<br />
3.6<br />
Problem Solving 3.29 Solve<br />
Problem 29 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
9 of 43<br />
Slide<br />
10 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.6<br />
SAMPLE PROBLEM 3.6<br />
Slide<br />
11 of 43<br />
Slide<br />
12 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
2
SAMPLE PROBLEM<br />
3.6<br />
Practice Problems for Sample Problem 3.6<br />
For Sample Problem 3.6<br />
Problem Solving 3.30 Solve<br />
Problem 30 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
13 of 43<br />
Slide<br />
14 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.3<br />
Conversion Problems > Converting Between Units<br />
3.3<br />
Conversion Problems > Converting Between Units<br />
Converting Between Units<br />
What types of problems are easily<br />
solved by using dimensional<br />
analysis?<br />
Problems in which a measurement<br />
with one unit is converted to an<br />
equivalent measurement with another<br />
unit are easily solved using<br />
dimensional analysis.<br />
Slide<br />
15 of 43<br />
Slide<br />
16 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.7<br />
SAMPLE PROBLEM 3.7<br />
Slide<br />
17 of 43<br />
Slide<br />
18 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3
SAMPLE PROBLEM<br />
3.7<br />
SAMPLE PROBLEM<br />
3.7<br />
Slide<br />
19 of 43<br />
Slide<br />
20 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Practice Problems for Sample Problem 3.7<br />
3.3<br />
Conversion Problems > Converting Between Units<br />
Multistep Problems<br />
When converting between units, it is often<br />
necessary to use more than one conversion<br />
factor. Sample problem 3.8 illustrates the use of<br />
multiple conversion factors.<br />
Problem Solving 3.33 Solve<br />
Problem 33 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
21 of 43<br />
Slide<br />
22 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.8<br />
SAMPLE PROBLEM 3.8<br />
Slide<br />
23 of 43<br />
Slide<br />
24 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
4
SAMPLE PROBLEM<br />
3.8<br />
SAMPLE PROBLEM<br />
3.8<br />
I don’t usually leave the “Evaluate” items in the<br />
PowerPoint BUT in this case, it is useful:<br />
Please think a moment about your answers in<br />
this (class or anywhere) to problems to make<br />
sure your conclusion seems at least logical.<br />
Slide<br />
25 of 43<br />
Slide<br />
26 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Practice Problems for Sample Problem 3.8<br />
3.3 Conversion Problems > Converting Between Units<br />
Converting Complex Units<br />
Many common measurements are expressed<br />
as a ratio of two units. If you use dimensional<br />
analysis, converting these complex units is just<br />
as easy as converting single units. It will just<br />
take multiple steps to arrive at an answer.<br />
Problem Solving 3.35 Solve<br />
Problem 35 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
27 of 43<br />
Slide<br />
28 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM<br />
3.9<br />
SAMPLE PROBLEM 3.9<br />
Slide<br />
29 of 43<br />
Slide<br />
30 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
5
SAMPLE PROBLEM<br />
3.9<br />
SAMPLE PROBLEM<br />
3.9<br />
Slide<br />
31 of 43<br />
Slide<br />
32 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Practice Problems for Sample Problem 3.9<br />
Section Assessment<br />
Assess students’ understanding<br />
of the concepts in Section 3.3<br />
Continue to:<br />
Section Quiz<br />
-or-<br />
Launch:<br />
Problem-Solving 3.37 Solve<br />
Problem 37 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
33 of 43<br />
Slide<br />
34 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.3 Section Quiz<br />
3.3 Section Quiz<br />
1. 1 Mg = 1000 kg. Which of the following would<br />
be a correct conversion factor for this<br />
relationship?<br />
a. × 1000.<br />
b. × 1/1000.<br />
c. ÷ 1000.<br />
d. 1000 kg/1Mg.<br />
Slide<br />
35 of 43<br />
2. The conversion factor used to convert joules to<br />
calories changes<br />
a. the quantity of energy measured but not the<br />
numerical value of the measurement.<br />
b. neither the numerical value of the measurement<br />
nor the quantity of energy measured.<br />
c. the numerical value of the measurement but not<br />
the quantity of energy measured.<br />
d. both the numerical value of the measurement and<br />
the quantity of energy measured.<br />
Slide<br />
36 of 43<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
6
3.3 Section Quiz<br />
3. How many µ g are in 0.0134 g?<br />
a. 1.34 × 10 –4<br />
b. 1.34 × 10 –6<br />
c. 1.34 × 10 6<br />
3.3 Section Quiz<br />
4. Express the density 5.6 g/cm 3 in kg/m 3 .<br />
a. 5.6 × 10 6 kg/m 3<br />
b. 5.6 × 10 3 kg/m 3<br />
c. 0.56 kg/m 3<br />
d. 0.0056 kg/m 3<br />
d. 1.34 × 10 4 © Copyright Pearson Prentice Hall<br />
Slide<br />
37 of 43<br />
Slide<br />
38 of 43<br />
© Copyright Pearson Prentice Hall<br />
END OF SHOW<br />
7
3.4<br />
Density > Determining Density<br />
3.4<br />
Density > Determining Density<br />
Determining Density<br />
Density is the ratio of the mass of an object to<br />
its volume.<br />
What determines the density of a<br />
substance?<br />
Slide<br />
1 of 25<br />
Slide<br />
2 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.4<br />
Density > Determining Density<br />
3.4<br />
Density > Determining Density<br />
Each of these 10-g samples has a different<br />
volume because the densities vary.<br />
Density is an intensive property that<br />
depends only on the composition of a<br />
substance, not on the size of the<br />
sample.<br />
Slide<br />
3 of 25<br />
Slide<br />
4 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.4<br />
Density > Determining Density<br />
Density > Determining Density<br />
The density of corn oil is<br />
less than the density of<br />
corn syrup. For that<br />
reason, the oil floats on<br />
top of the syrup.<br />
Simulation 1<br />
Rank materials according to their densities.<br />
Slide<br />
5 of 25<br />
Slide<br />
6 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
1
3.4<br />
Density > Density and Temperature<br />
SAMPLE PROBLEM 3.10<br />
Experiments show that the volume of most<br />
substances increases as the temperature<br />
increases. Meanwhile, the mass remains<br />
the same. Thus, the density must change.<br />
The density of a substance generally<br />
decreases as its temperature<br />
increases.<br />
Slide<br />
7 of 25<br />
Slide<br />
8 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM 3.10<br />
SAMPLE PROBLEM 3.10<br />
Slide<br />
9 of 25<br />
Slide<br />
10 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM 3.10<br />
Practice Problems for Sample Problem 3.10<br />
Problem Solving 3.47 Solve<br />
Problem 47 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
11 of 25<br />
Slide<br />
12 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
2
SAMPLE PROBLEM 3.11<br />
SAMPLE PROBLEM 3.11<br />
Slide<br />
13 of 25<br />
Slide<br />
14 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
SAMPLE PROBLEM 3.11<br />
SAMPLE PROBLEM 3.11<br />
Slide<br />
15 of 25<br />
Slide<br />
16 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
Practice Problems<br />
for Sample Problem 3.11<br />
Section Assessment<br />
Assess students’ understanding<br />
of the concepts in Section 3.4<br />
Continue to:<br />
Section Quiz<br />
-or-<br />
Launch:<br />
Problem Solving 3.48 Solve<br />
Problem 48 with the help of an<br />
interactive guided tutorial.<br />
Slide<br />
17 of 25<br />
Slide<br />
18 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3
3.4 Section Quiz<br />
1. If 50.0 mL of corn syrup have a mass of 68.7<br />
g, the density of the corn syrup is<br />
a. 0.737 g/mL.<br />
b. 0.727 g/mL.<br />
c. 1.36 g/mL.<br />
d. 1.37 g/mL.<br />
3.4 Section Quiz<br />
2. What is the volume of a pure gold coin that<br />
has a mass of 38.6 g? The density of gold is<br />
19.3 g/cm 3 .<br />
a. 0.500 cm 3<br />
b. 2.00 cm 3<br />
c. 38.6 cm 3<br />
d. 745 cm 3<br />
Slide<br />
19 of 25<br />
Slide<br />
20 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
3.4 Section Quiz<br />
3. As the temperature increases, the density of<br />
most substances<br />
a. increases.<br />
Concept Map 3<br />
Density > Concept map<br />
Solve the Concept Map with the help of an<br />
interactive guided tutorial.<br />
b. decreases.<br />
c. remains the same.<br />
d. increases at first and then decreases.<br />
Slide<br />
21 of 25<br />
Slide<br />
22 of 25<br />
© Copyright Pearson Prentice Hall<br />
© Copyright Pearson Prentice Hall<br />
END OF SHOW<br />
4