Basics of Pulse Combustion Technology
Basics of Pulse Combustion Technology
Basics of Pulse Combustion Technology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
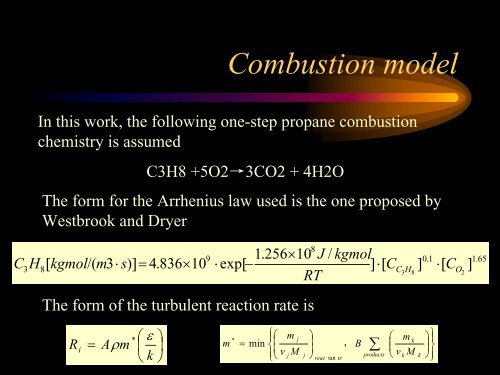
<strong>Combustion</strong> modelCIn this work, the following one-step propane combustionchemistry is assumedC3H8 +5O2→3CO2 + 4H2OThe form for the Arrhenius law used is the one proposed byWestbrook and Dryer1.256×10 JRT/ kgmol890.1 1.653H8kgmol/(m3⋅ s)]= 4.836×10 ⋅ exp[ −] ⋅[C C H] ⋅[C ][3 8 O 2The form <strong>of</strong> the turbulent reaction rate isR i=⎛ ε ⎞Aρ m* ⎪⎧⎛ m ⎞⎪⎫*j⎛ ⎞⎜ ⎟⎨⎜⎟mkm = min, B⎜⎟∑ ⎬⎝ k ⎠⎪⎩ ⎝ vjMj ⎠productsreac ts⎝ vkMtank ⎠⎪ ⎭