n - Wits Structural Chemistry
n - Wits Structural Chemistry
n - Wits Structural Chemistry
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
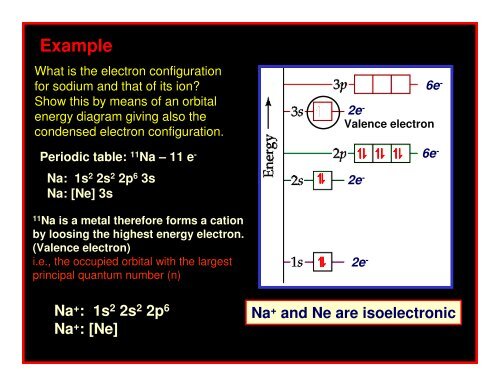
ExampleWhat is the electron configurationfor sodium and that of its ion?Show this by means of an orbitalenergy diagram giving also thecondensed electron configuration.Periodic table: 11 Na – 11 e -Na: 1s 2 2s 2 2p 6 3sNa: [Ne] 3s11Na is a metal therefore forms a cationby loosing the highest energy electron.(Valence electron)i.e., the occupied orbital with the largestprincipal quantum number (n)↿↿⇂↿⇂2e - 6e -Valence electron↿↿↿ ⇂⇂⇂2e - 6e -2e -Na + : 1s 2 2s 2 2p 6Na + : [Ne]Na + and Ne are isoelectronic