Graham's Law
Graham's Law
Graham's Law
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
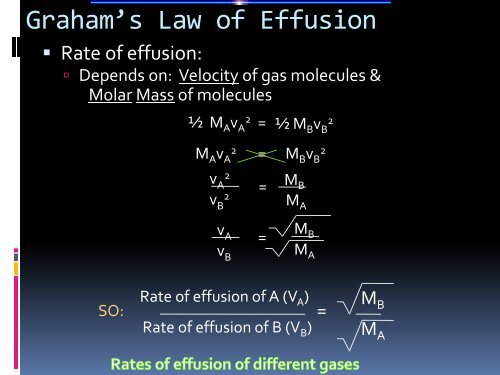
Graham’s <strong>Law</strong> of Effusion• Rate of effusion: Depends on: Velocity of gas molecules &Molar Mass of molecules½ M A v A2 = ½ M B v B2M A v A2= M B v B2v A2v B2v Av B==M BM AM BM ASO:Rate of effusion of A (V A )Rate of effusion of B (V B )=M BM A