Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Problem 6 Atomic and molecular orbitals - PianetaChimica.it
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
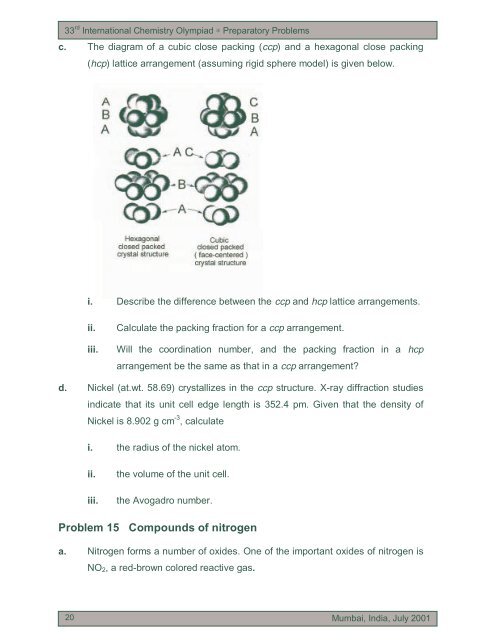
33 rd International Chemistry Olympiad ∗ Preparatory <strong>Problem</strong>sc. The diagram of a cubic close packing (ccp) <strong>and</strong> a hexagonal close packing(hcp) lattice arrangement (assuming rigid sphere model) is given below.i. Describe the difference between the ccp <strong>and</strong> hcp lattice arrangements.ii.iii.Calculate the packing fraction for a ccp arrangement.Will the coordination number, <strong>and</strong> the packing fraction in a hcparrangement be the same as that in a ccp arrangement?d. Nickel (at.wt. 58.69) crystallizes in the ccp structure. X-ray diffraction studiesindicate that <strong>it</strong>s un<strong>it</strong> cell edge length is 352.4 pm. Given that the dens<strong>it</strong>y ofNickel is 8.902 g cm -3 , calculatei. the radius of the nickel atom.ii.iii.the volume of the un<strong>it</strong> cell.the Avogadro number.<strong>Problem</strong> 15 Compounds of n<strong>it</strong>rogena. N<strong>it</strong>rogen forms a number of oxides. One of the important oxides of n<strong>it</strong>rogen isNO 2 , a red-brown colored reactive gas.2020Mumbai, India, July 2001