Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
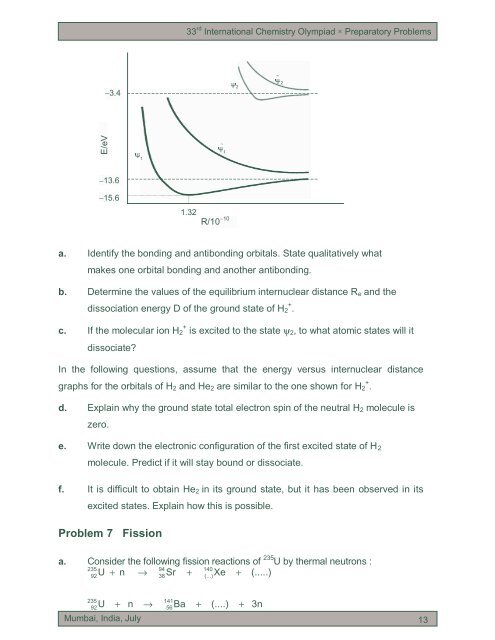
33 rd International Chemistry Olympiad ∗ Preparatory <strong>Problem</strong>s−3.4 2~2E/eV 1~ 1−13.6−15.61.32R/10 −10a. Identify the bonding <strong>and</strong> antibonding <strong>orb<strong>it</strong>als</strong>. State qual<strong>it</strong>atively whatmakes one orb<strong>it</strong>al bonding <strong>and</strong> another antibonding.b. Determine the values of the equilibrium internuclear distance R e <strong>and</strong> thedissociation energy D of the ground state of H + 2 .c. If the <strong>molecular</strong> ion H + 2 is exc<strong>it</strong>ed to the state ψ2, to what atomic states will <strong>it</strong>dissociate?In the following questions, assume that the energy versus internuclear distancegraphs for the <strong>orb<strong>it</strong>als</strong> of H 2 <strong>and</strong> He 2 are similar to the one shown for H + 2 .d. Explain why the ground state total electron spin of the neutral H 2 molecule iszero.e. Wr<strong>it</strong>e down the electronic configuration of the first exc<strong>it</strong>ed state of H 2molecule. Predict if <strong>it</strong> will stay bound or dissociate.f. It is difficult to obtain He 2 in <strong>it</strong>s ground state, but <strong>it</strong> has been observed in <strong>it</strong>sexc<strong>it</strong>ed states. Explain how this is possible.<strong>Problem</strong> 7 Fissiona. Consider the following fission reactions of 235 U by thermal neutrons :23594140U + n → Sr + Xe + (.....)9238(...)23592U+ n →Mumbai, India, July14156Ba+(....)+3n13 13