Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Problem 6 Atomic and molecular orbitals - PianetaChimica.it
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
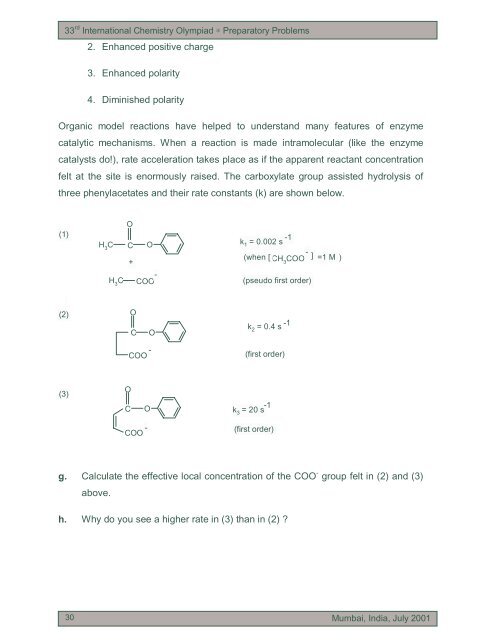
33 rd International Chemistry Olympiad ∗ Preparatory <strong>Problem</strong>s2. Enhanced pos<strong>it</strong>ive charge3. Enhanced polar<strong>it</strong>y4. Diminished polar<strong>it</strong>yOrganic model reactions have helped to underst<strong>and</strong> many features of enzymecatalytic mechanisms. When a reaction is made intra<strong>molecular</strong> (like the enzymecatalysts do!), rate acceleration takes place as if the apparent reactant concentrationfelt at the s<strong>it</strong>e is enormously raised. The carboxylate group assisted hydrolysis ofthree phenylacetates <strong>and</strong> their rate constants (k) are shown below.(1)OH 3C C+OH 3C-COO-1k 1 = 0.002 s-(when [ CH 3COO ] =1 M )(pseudo first order)(2)OCOk 2= 0.4 s-1COO -(first order)(3)OC Ok 3= 20 s -1COO - (first order)g. Calculate the effective local concentration of the COO - group felt in (2) <strong>and</strong> (3)above.h. Why do you see a higher rate in (3) than in (2) ?3030Mumbai, India, July 2001