4 Behavior of Gases
4 Behavior of Gases
4 Behavior of Gases
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
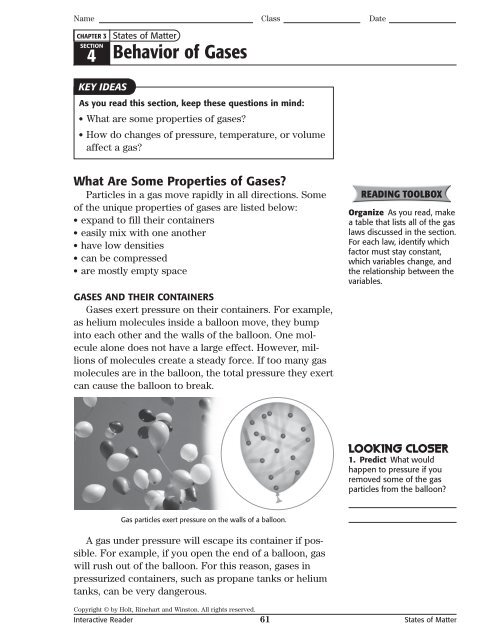
Name Class DateCHAPTER 3States <strong>of</strong> Matter4 <strong>Behavior</strong> <strong>of</strong> <strong>Gases</strong>SECTIONKEY IDEASAs you read this section, keep these questions in mind:• What are some properties <strong>of</strong> gases?• How do changes <strong>of</strong> pressure, temperature, or volumeaffect a gas?What Are Some Properties <strong>of</strong> <strong>Gases</strong>?Particles in a gas move rapidly in all directions. Some<strong>of</strong> the unique properties <strong>of</strong> gases are listed below:• expand to fill their containers• easily mix with one another• have low densities• can be compressed• are mostly empty spaceGASES AND THEIR CONTAINERS<strong>Gases</strong> exert pressure on their containers. For example,as helium molecules inside a balloon move, they bumpinto each other and the walls <strong>of</strong> the balloon. One moleculealone does not have a large effect. However, millions<strong>of</strong> molecules create a steady force. If too many gasmolecules are in the balloon, the total pressure they exertcan cause the balloon to break.READING TOOLBOXOrganize As you read, makea table that lists all <strong>of</strong> the gaslaws discussed in the section.For each law, identify whichfactor must stay constant,which variables change, andthe relationship between thevariables.1. Predict What wouldhappen to pressure if youremoved some <strong>of</strong> the gasparticles from the balloon?Gas particles exert pressure on the walls <strong>of</strong> a balloon.A gas under pressure will escape its container if possible.For example, if you open the end <strong>of</strong> a balloon, gaswill rush out <strong>of</strong> the balloon. For this reason, gases inpressurized containers, such as propane tanks or heliumtanks, can be very dangerous.Copyright © by Holt, Rinehart and Winston. All rights reserved.Interactive Reader 61 States <strong>of</strong> Matter
Name Class DateSECTION 4<strong>Behavior</strong> <strong>of</strong> <strong>Gases</strong> continuedWhat Are the Gas Laws?<strong>Gases</strong> behave differently than solids or liquids do. Forexample, the volume <strong>of</strong> a gas can change due to pressure,but the volume <strong>of</strong> a solid or liquid generally cannot. Thegas laws describe how variables such as pressure, volume,and temperature affect the behavior <strong>of</strong> gases. Thegas laws will help you understand and predict the behavior<strong>of</strong> gases in specific situations.READING CHECK2. Identify Boyle’s lawdescribes the relationshipbetween which two variables?PRESSURE AND VOLUMEA diver is swimming at a depth <strong>of</strong> 10 m below sealevel. An air bubble escapes from her mouthpiece. Asthe bubble rises to the surface, it gets bigger. When thebubble reaches the water’s surface, its volume is doubleits original size.This example shows the relationship between the volumeand pressure <strong>of</strong> a gas, also known as Boyle’s law.Boyle’s law is true for almost any gas, if temperatureand amount <strong>of</strong> gas are constant, or unchanged.Boyle’s LawFor a certain amount <strong>of</strong> gas at a constant temperature, thevolume <strong>of</strong> a gas decreases as the gas’s pressure increases.Likewise, the volume <strong>of</strong> a gas increases as the gas’s pressuredecreases.In mathematical terms:Boyle’s Law(initial pressure)(initial volume) = (final pressure)(final volume)P 1V 1= P 2V 2The figure below illustrates Boyle’s law. Both pistonscontain the same amount <strong>of</strong> gas at the same temperature.3. Identify What happens tovolume as pressuredecreases?If you lift the piston, pressuredecreases. The gas particlesspread farther apart, and thevolume increases.If you push the piston, pressureincreases. The gas particles arepushed closer together, and thevolume decreases.Copyright © by Holt, Rinehart and Winston. All rights reserved.Interactive Reader 62 States <strong>of</strong> Matter
Name Class DateSECTION 4<strong>Behavior</strong> <strong>of</strong> <strong>Gases</strong> continuedAPPLYING BOYLE’S LAWYou can use Boyle’s law to predict changes in the pressureor volume <strong>of</strong> a gas. Remember that Boyle’s law istrue only when the temperature and amount <strong>of</strong> gas do notchange.A balloon has a volume <strong>of</strong> 7.5 L at 100.0 kPa. As theballoon rises in the atmosphere, the gas inside expandsto a volume <strong>of</strong> 11 L. Assume the balloon is at a constanttemperature and the amount <strong>of</strong> gas does not change.What is the pressure when the volume is 11 L?READING CHECK4. Identify Under whatconditions does Boyle’s lawapply?The ballon on the left has a volume <strong>of</strong> 7.5 L and a pressure <strong>of</strong> 100 kPa. As the balloonrises, it becomes larger. The balloon’s new volume is 11 L. The temperatureand number <strong>of</strong> molecules inside the balloon stay the same.Step 1: List the given and unknownvalues.Step 2: Write the equation andrearrange to solve for the unknown.Given:V 1= 7.5 LP 1= 100.0 kPaV 2= 11 LP 1V 1= P 2V 2P 2= _P V 1 1V 2Step 3: Insert the known values and (100.0 kPa)(7.5 L)solve for the unknown value. P 2= __11 LP 2= 68 kPaUnknown:P 2MathSkills5. Calculate A 300 mLsample <strong>of</strong> hydrogen gas isat a pressure <strong>of</strong> 0.500 kPa.If the pressure increases to0.750 kPa, what will be thefinal volume <strong>of</strong> the sample?Assume that temperaturestays constant.PRESSURE AND TEMPERATURERecall that temperature is a measure <strong>of</strong> the averagekinetic energy <strong>of</strong> particles. As the particles <strong>of</strong> a substancemove faster, the substance’s temperature increases. Theparticles bump into each other and the sides <strong>of</strong> the containermore <strong>of</strong>ten, which increases pressure. Thus, astemperature increases, pressure increases. This is knownas Gay-Lussac’s law.Copyright © by Holt, Rinehart and Winston. All rights reserved.Interactive Reader 63 States <strong>of</strong> Matter
Name Class DateSECTION 4<strong>Behavior</strong> <strong>of</strong> <strong>Gases</strong> continuedGay-Lussac’s LawWhen volume is constant, the pressure <strong>of</strong> a gasincreases as temperature increases. Pressuredecreases as temperature decreases.In other words, the pressure and temperature <strong>of</strong> a gasare directly related. As one changes, the other changes inthe same direction.6. Compare How are the relationshipsbetween variablesdescribed in Gay-Lussac’s lawand Charles’s law similar?TEMPERATURE AND VOLUMELike the temperature and pressure <strong>of</strong> a gas, the temperatureand volume <strong>of</strong> a gas are directly related. Thisrelationship is described in Charles’s Law.Charles’s LawWhen the amount <strong>of</strong> a gas and pressure areconstant, the volume <strong>of</strong> a gas increases as itstemperature increases. Likewise, as volumedecreases, temperature decreases.The figure below illustrates Charles’s Law. Both pistonshave the same amount <strong>of</strong> gas at the same pressure.When temperature decreases, thegas particles move more slowly andvolume decreases.When temperature increases, the gasparticles move faster and volumeincreases.The following experiment also illustrates Charles’s law.7. Identify What two factorsdid not change during theexperiment?Air-filled balloons are putinto liquid nitrogen.The low temperature<strong>of</strong> the liquid nitrogenmakes the volumes <strong>of</strong>the air in the balloonssmaller.”When the balloons areremoved from the liquidnitrogen, their temperatureincreases. Thevolume <strong>of</strong> each balloonincreases to its originalvolume.Copyright © by Holt, Rinehart and Winston. All rights reserved.Interactive Reader 64 States <strong>of</strong> Matter
Name Class DateSECTION 4<strong>Behavior</strong> <strong>of</strong> <strong>Gases</strong> continuedHow Can Graphs Illustrate the Gas Laws?You can use graphs to show how temperature, pressure,and volume affect gases. A graph can show therelationship between two factors. For example, the graphcan show if a relationship is direct or inverse. In a directrelationship, the two variables change in the same direction.In an inverse relationship, the variables change inopposite directions. In the graph below, temperature andvolume have a direct relationship.Volume (L)Volume versus Temperature fora Gas at a Constant Pressure0.7000.6000.5000.4000.3000.200GraphingSkills8. Analyze Is the relationshipshown in this graphdirect or inverse? How doyou know?0.1000.0000 100 200 300Temperature (K)The shape <strong>of</strong> the line in a graph also describes therelationship. If a graph is a straight line, such as thegraph above, one variable is directly or inverselyproportional to the other. In a proportional relationship,the variables stay in the same ratio to each other as theirvalues change. If a graph is a curve, one variable is notproportional to the other. This means that the variablesdo not stay in the same ratio to each other as their valueschange.Volume (L)Volume versus Pressurefor a Gas at a Constant Temperature0.5000.4000.3000.2000.1009. Analyze Is this relationshipproportional? Explainyour answer.10. Identify Which gas lawdoes this graph represent?11. Infer Is the relationshipbetween the variables director inverse? Explain youranswer.0.0000 100 200 300 400Pressure (kPa)Copyright © by Holt, Rinehart and Winston. All rights reserved.Interactive Reader 65 States <strong>of</strong> Matter
Name Class DateSection 4 ReviewSECTION VOCABULARYgas laws the laws that state the mathematicalrelationships between the volume, temperature,pressure, and quantity <strong>of</strong> a gas1. Identify How do gas particles exert pressure on their container?2. Apply Concepts Chandra notices that her bicycle tires have higher pressure duringthe hot summer than during the cold winter. Which gas law explains her observation?Explain your answer.3. Predict What would happen eventually to a balloon sitting in a sunny window?Which gas law predicts this?4. Describe In Boyle’s law, what is the relationship between pressure and volume?5. Graph Relationships In the space below, create a graph showing the proportionalrelationship between temperature and pressure described by Gay-Lussac’s law.Be sure to label the axes <strong>of</strong> your graph and give your graph a title.Copyright © by Holt, Rinehart and Winston. All rights reserved.Interactive Reader 66 States <strong>of</strong> Matter