single rp-hplc method for the estimation of losartan ... - Ijsidonline.info
single rp-hplc method for the estimation of losartan ... - Ijsidonline.info
single rp-hplc method for the estimation of losartan ... - Ijsidonline.info
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
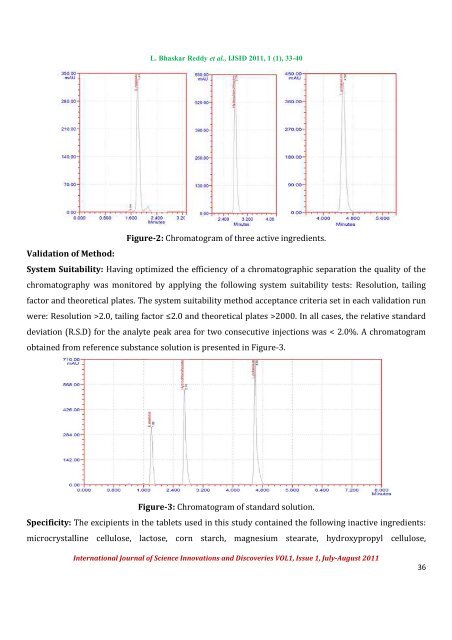
L. Bhaskar Reddy et al., IJSID 2011, 1 (1), 33-40Figure-2: Chromatogram <strong>of</strong> three active ingredients.Validation <strong>of</strong> Method:System Suitability: Having optimized <strong>the</strong> efficiency <strong>of</strong> a chromatographic separation <strong>the</strong> quality <strong>of</strong> <strong>the</strong>chromatography was monitored by applying <strong>the</strong> following system suitability tests: Resolution, tailingfactor and <strong>the</strong>oretical plates. The system suitability <strong>method</strong> acceptance criteria set in each validation runwere: Resolution >2.0, tailing factor ≤2.0 and <strong>the</strong>oretical plates >2000. In all cases, <strong>the</strong> relative standarddeviation (R.S.D) <strong>for</strong> <strong>the</strong> analyte peak area <strong>for</strong> two consecutive injections was < 2.0%. A chromatogramobtained from reference substance solution is presented in Figure-3.Figure-3: Chromatogram <strong>of</strong> standard solution.Specificity: The excipients in <strong>the</strong> tablets used in this study contained <strong>the</strong> following inactive ingredients:microcrystalline cellulose, lactose, corn starch, magnesium stearate, hydroxypropyl cellulose,International Journal <strong>of</strong> Science Innovations and Discoveries VOL1, Issue 1, July-August 201136