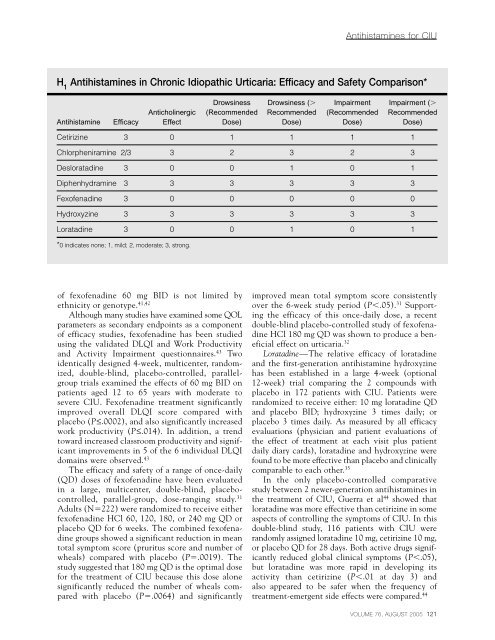
<strong>Antihistam<strong>in</strong>es</strong> for CIUH 1<strong>Antihistam<strong>in</strong>es</strong> <strong>in</strong> Chronic Idiopathic Urticaria: Efficacy and Safety Comparison*Drows<strong>in</strong>ess Drows<strong>in</strong>ess ( Impairment Impairment (Antichol<strong>in</strong>ergic (Recommended Recommended (Recommended RecommendedAntihistam<strong>in</strong>e Efficacy Effect Dose) Dose) Dose) Dose)Cetiriz<strong>in</strong>e 3 0 1 1 1 1Chlorpheniram<strong>in</strong>e 2/3 3 2 3 2 3Desloratad<strong>in</strong>e 3 0 0 1 0 1Diphenhydram<strong>in</strong>e 3 3 3 3 3 3Fex<strong>of</strong>enad<strong>in</strong>e 3 0 0 0 0 0Hydroxyz<strong>in</strong>e 3 3 3 3 3 3Loratad<strong>in</strong>e 3 0 0 1 0 1*0 <strong>in</strong>dicates none; 1, mild; 2, moderate; 3, strong.<strong>of</strong> fex<strong>of</strong>enad<strong>in</strong>e 60 mg BID is not limited byethnicity or genotype. 41,42Although many studies have exam<strong>in</strong>ed some QOLparameters as secondary endpo<strong>in</strong>ts as a component<strong>of</strong> efficacy studies, fex<strong>of</strong>enad<strong>in</strong>e has been studiedus<strong>in</strong>g <strong>the</strong> validated DLQI and Work Productivityand Activity Impairment questionnaires. 43 Twoidentically designed 4-week, multicenter, randomized,double-bl<strong>in</strong>d, placebo-controlled, parallelgrouptrials exam<strong>in</strong>ed <strong>the</strong> effects <strong>of</strong> 60 mg BID onpatients aged 12 to 65 years with moderate tosevere CIU. Fex<strong>of</strong>enad<strong>in</strong>e treatment significantlyimproved overall DLQI score compared withplacebo (P≤.0002), and also significantly <strong>in</strong>creasedwork productivity (P≤.014). In addition, a trendtoward <strong>in</strong>creased classroom productivity and significantimprovements <strong>in</strong> 5 <strong>of</strong> <strong>the</strong> 6 <strong>in</strong>dividual DLQIdoma<strong>in</strong>s were observed. 43The efficacy and safety <strong>of</strong> a range <strong>of</strong> once-daily(QD) doses <strong>of</strong> fex<strong>of</strong>enad<strong>in</strong>e have been evaluated<strong>in</strong> a large, multicenter, double-bl<strong>in</strong>d, placebocontrolled,parallel-group, dose-rang<strong>in</strong>g study. 31Adults (N222) were randomized to receive ei<strong>the</strong>rfex<strong>of</strong>enad<strong>in</strong>e HCl 60, 120, 180, or 240 mg QD orplacebo QD for 6 weeks. The comb<strong>in</strong>ed fex<strong>of</strong>enad<strong>in</strong>egroups showed a significant reduction <strong>in</strong> meantotal symptom score (pruritus score and number <strong>of</strong>wheals) compared with placebo (P.0019). Thestudy suggested that 180 mg QD is <strong>the</strong> optimal dosefor <strong>the</strong> treatment <strong>of</strong> CIU because this dose alonesignificantly reduced <strong>the</strong> number <strong>of</strong> wheals comparedwith placebo (P.0064) and significantlyimproved mean total symptom score consistentlyover <strong>the</strong> 6-week study period (P.05). 31 Support<strong>in</strong>g<strong>the</strong> efficacy <strong>of</strong> this once-daily dose, a recentdouble-bl<strong>in</strong>d placebo-controlled study <strong>of</strong> fex<strong>of</strong>enad<strong>in</strong>eHCl 180 mg QD was shown to produce a beneficialeffect on urticaria. 32Loratad<strong>in</strong>e—The relative efficacy <strong>of</strong> loratad<strong>in</strong>eand <strong>the</strong> first-generation antihistam<strong>in</strong>e hydroxyz<strong>in</strong>ehas been established <strong>in</strong> a large 4-week (optional12-week) trial compar<strong>in</strong>g <strong>the</strong> 2 compounds withplacebo <strong>in</strong> 172 patients with CIU. Patients wererandomized to receive ei<strong>the</strong>r: 10 mg loratad<strong>in</strong>e QDand placebo BID; hydroxyz<strong>in</strong>e 3 times daily; orplacebo 3 times daily. As measured by all efficacyevaluations (physician and patient evaluations <strong>of</strong><strong>the</strong> effect <strong>of</strong> treatment at each visit plus patientdaily diary cards), loratad<strong>in</strong>e and hydroxyz<strong>in</strong>e werefound to be more effective than placebo and cl<strong>in</strong>icallycomparable to each o<strong>the</strong>r. 35In <strong>the</strong> only placebo-controlled comparativestudy between 2 newer-generation antihistam<strong>in</strong>es <strong>in</strong><strong>the</strong> treatment <strong>of</strong> CIU, Guerra et al 44 showed thatloratad<strong>in</strong>e was more effective than cetiriz<strong>in</strong>e <strong>in</strong> someaspects <strong>of</strong> controll<strong>in</strong>g <strong>the</strong> symptoms <strong>of</strong> CIU. In thisdouble-bl<strong>in</strong>d study, 116 patients with CIU wererandomly assigned loratad<strong>in</strong>e 10 mg, cetiriz<strong>in</strong>e 10 mg,or placebo QD for 28 days. Both active drugs significantlyreduced global cl<strong>in</strong>ical symptoms (P.05),but loratad<strong>in</strong>e was more rapid <strong>in</strong> develop<strong>in</strong>g itsactivity than cetiriz<strong>in</strong>e (P.01 at day 3) andalso appeared to be safer when <strong>the</strong> frequency <strong>of</strong>treatment-emergent side effects were compared. 44VOLUME 76, AUGUST 2005 121
<strong>Antihistam<strong>in</strong>es</strong> for CIUDesloratad<strong>in</strong>e—Desloratad<strong>in</strong>e is <strong>the</strong> major activemetabolite <strong>of</strong> loratad<strong>in</strong>e, which has been available<strong>in</strong> <strong>the</strong> United States s<strong>in</strong>ce 2002 for <strong>the</strong> treatment <strong>of</strong>CIU. The efficacy <strong>of</strong> <strong>the</strong> drug has been evaluated <strong>in</strong>2 major randomized controlled cl<strong>in</strong>ical trials. 37,38R<strong>in</strong>g et al 37 reported that desloratad<strong>in</strong>e exhibitedsuperior efficacy compared with placebo <strong>in</strong> amulticenter, randomized, double-bl<strong>in</strong>d trial <strong>of</strong>190 patients with a history <strong>of</strong> CIU. Patients wereassigned to receive ei<strong>the</strong>r desloratad<strong>in</strong>e 5 mg QDor placebo QD for 6 weeks. The active treatmentwas superior to placebo at reduc<strong>in</strong>g pruritus andoverall symptoms after <strong>the</strong> first dose and throughout<strong>the</strong> 6-week study. 37 Similarly, <strong>the</strong>rapeuticresponse and global CIU status, as well as QOLmeasures such as <strong>in</strong>terference with sleep, wereimproved with desloratad<strong>in</strong>e compared withplacebo throughout <strong>the</strong> study period. 37 Us<strong>in</strong>g <strong>the</strong>same dose (5 mg QD), a fur<strong>the</strong>r 6-week placebocontrolledstudy <strong>of</strong> desloratad<strong>in</strong>e <strong>in</strong>dicated <strong>the</strong>effectiveness <strong>of</strong> this agent at reliev<strong>in</strong>g CIU symptoms.38 Over <strong>the</strong> study period, <strong>the</strong> mean total CIUsymptom score was significantly improved comparedwith placebo, as were <strong>the</strong> <strong>in</strong>dividual scores <strong>of</strong>pruritus, number <strong>of</strong> hives, and <strong>the</strong> size <strong>of</strong> <strong>the</strong> largesthive. Interference with sleep was reduced and performance<strong>of</strong> daily activities was improved withdesloratad<strong>in</strong>e. These statistically and cl<strong>in</strong>ically significantimprovements were seen with<strong>in</strong> <strong>the</strong> first24 hours <strong>of</strong> treatment and were susta<strong>in</strong>ed throughout<strong>the</strong> 6-week treatment period. 38Cetiriz<strong>in</strong>e—As with loratad<strong>in</strong>e, cetiriz<strong>in</strong>e hasbeen shown to be as effective as first-generationhydroxyz<strong>in</strong>e at reliev<strong>in</strong>g <strong>the</strong> symptoms <strong>of</strong> CIU. 40For example, a 4-week, multicenter, randomized,double-bl<strong>in</strong>d, double-dummy trial <strong>in</strong>vestigated <strong>the</strong>efficacy and safety <strong>of</strong> cetiriz<strong>in</strong>e 10 mg QD andhydroxyz<strong>in</strong>e 25 mg 3 times daily compared withplacebo <strong>in</strong> patients with CIU. Patients <strong>in</strong> <strong>the</strong> cetiriz<strong>in</strong>eand hydroxyz<strong>in</strong>e groups showed significantreductions dur<strong>in</strong>g weeks 1, 2, 3, and 4 <strong>in</strong> <strong>the</strong> numberand size <strong>of</strong> lesions and <strong>in</strong> <strong>the</strong> severity <strong>of</strong> prurituscompared with patients who received placebo.In addition, physician and patient evaluations at<strong>the</strong> end <strong>of</strong> week 4 revealed an improvement <strong>in</strong>urticarial symptoms for <strong>the</strong> cetiriz<strong>in</strong>e and hydroxyz<strong>in</strong>egroups compared with <strong>the</strong> placebo group. 40All 4 newer-generation H 1antihistam<strong>in</strong>es (fex<strong>of</strong>enad<strong>in</strong>e,loratad<strong>in</strong>e, desloratad<strong>in</strong>e, and cetiriz<strong>in</strong>e)have been shown to be superior to placebo at treat<strong>in</strong>g<strong>the</strong> symptoms <strong>of</strong> CIU, and both loratad<strong>in</strong>e andcetiriz<strong>in</strong>e have been proven to be as effective asfirst-generation hydroxyz<strong>in</strong>e. 35,40 Although no trialshave evaluated fex<strong>of</strong>enad<strong>in</strong>e and desloratad<strong>in</strong>e comparedwith hydroxyz<strong>in</strong>e, comparisons demonstrat<strong>in</strong>gequivalence have been made with <strong>the</strong>ir parent compounds(loratad<strong>in</strong>e 35 and terfenad<strong>in</strong>e 45 ).There are few controlled studies <strong>in</strong> whichnewer-generation antihistam<strong>in</strong>es have been directlycompared, and <strong>the</strong>re is no evidence-based datademonstrat<strong>in</strong>g statistical superiority <strong>of</strong> one secondgenerationagent over ano<strong>the</strong>r <strong>in</strong> <strong>the</strong> treatment <strong>of</strong>CIU. For example, although a recent trial compared<strong>the</strong> efficacy <strong>of</strong> cetiriz<strong>in</strong>e with fex<strong>of</strong>enad<strong>in</strong>e,<strong>the</strong> results are weakened by <strong>the</strong> study design.Patients with CIU were randomized to ei<strong>the</strong>rcetiriz<strong>in</strong>e 10 mg (n52) or fex<strong>of</strong>enad<strong>in</strong>e 180 mg(n45); at 28 days, 51.9% (27) and 4.4% (2) <strong>of</strong>cetiriz<strong>in</strong>e and fex<strong>of</strong>enad<strong>in</strong>e patients, respectively,were symptom free (P.00001), while partialimprovement was experienced by 36.5% (19) <strong>of</strong>cetiriz<strong>in</strong>e patients and 42.2% (19) <strong>of</strong> fex<strong>of</strong>enad<strong>in</strong>epatients. 46 However, <strong>the</strong>re was no control group,basel<strong>in</strong>e symptom severity data were not provided,and <strong>the</strong> authors did not describe how <strong>the</strong>patients’ symptoms were assessed. 46 Therefore, adef<strong>in</strong>itive assessment <strong>of</strong> <strong>the</strong> relative efficacy <strong>of</strong>newer-generation antihistam<strong>in</strong>es cannot be achievedby review<strong>in</strong>g published trials alone.Anti-<strong>in</strong>flammatory PropertiesDue to <strong>the</strong> absence <strong>of</strong> well-designed placebocontrolledcomparisons <strong>of</strong> newer-generation antihistam<strong>in</strong>es,o<strong>the</strong>r properties have been exam<strong>in</strong>edto aid treatment comparisons. For example, it hasbeen suggested that some H 1-receptor antagonistsmay achieve anti-<strong>in</strong>flammatory effects <strong>in</strong> a cl<strong>in</strong>icalcontext, which could prove advantageous <strong>in</strong> <strong>the</strong>treatment <strong>of</strong> CIU because <strong>the</strong> disease is characterizedby tissue <strong>in</strong>flammation. 47To <strong>in</strong>vestigate <strong>the</strong> anti-<strong>in</strong>flammatory activity <strong>of</strong>fex<strong>of</strong>enad<strong>in</strong>e, an immunohistochemical evaluation<strong>of</strong> <strong>the</strong> agent was undertaken <strong>in</strong> patients with CIU. 48Twenty patients received fex<strong>of</strong>enad<strong>in</strong>e HCl 180 mgQD for 4 weeks; <strong>the</strong> expression <strong>of</strong> adhesionmolecules, mast cell proteases, and pro<strong>in</strong>flammatorycytok<strong>in</strong>es were evaluated before and after treatment,as were <strong>the</strong> patients’ assessments <strong>of</strong> urticarial symptoms.After treatment with fex<strong>of</strong>enad<strong>in</strong>e, significantdecreases <strong>in</strong> <strong>the</strong> expression <strong>of</strong> endo<strong>the</strong>lial leukocyteadhesion molecule-1 (P.02), vascular cell adhesionmolecule-1 (P.04), and tryptase (P.04) wereobserved, confirm<strong>in</strong>g <strong>the</strong> hypo<strong>the</strong>sis that fex<strong>of</strong>enad<strong>in</strong>ehas some anti-<strong>in</strong>flammatory properties.This study <strong>in</strong> humans must be put <strong>in</strong>to contextwith <strong>the</strong> numerous <strong>in</strong> vitro, ex vivo, and animalstudies that have been conducted <strong>in</strong> this area. A review<strong>of</strong> such data suggests that all newer-generationantihistam<strong>in</strong>es <strong>in</strong>hibit <strong>the</strong> release or generation <strong>of</strong>multiple <strong>in</strong>flammatory mediators, <strong>in</strong>clud<strong>in</strong>g IL-4,122 CUTIS ®