Sulfur BiogeochemistryâPast and Present
Sulfur BiogeochemistryâPast and Present
Sulfur BiogeochemistryâPast and Present
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
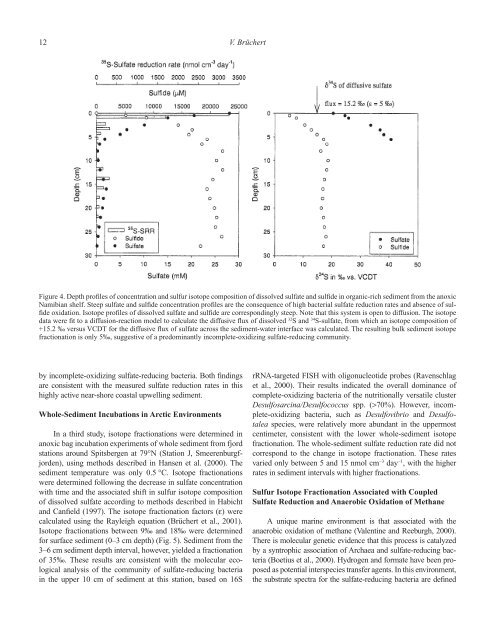
12 V. BrüchertFigure 4. Depth profiles of concentration <strong>and</strong> sulfur isotope composition of dissolved sulfate <strong>and</strong> sulfide in organic-rich sediment from the anoxicNamibian shelf. Steep sulfate <strong>and</strong> sulfide concentration profiles are the consequence of high bacterial sulfate reduction rates <strong>and</strong> absence of sulfideoxidation. Isotope profiles of dissolved sulfate <strong>and</strong> sulfide are correspondingly steep. Note that this system is open to diffusion. The isotopedata were fit to a diffusion-reaction model to calculate the diffusive flux of dissolved 32 S <strong>and</strong> 34 S-sulfate, from which an isotope composition of+15.2 ‰ versus VCDT for the diffusive flux of sulfate across the sediment-water interface was calculated. The resulting bulk sediment isotopefractionation is only 5‰, suggestive of a predominantly incomplete-oxidizing sulfate-reducing community.by incomplete-oxidizing sulfate-reducing bacteria. Both findingsare consistent with the measured sulfate reduction rates in thishighly active near-shore coastal upwelling sediment.Whole-Sediment Incubations in Arctic EnvironmentsIn a third study, isotope fractionations were determined inanoxic bag incubation experiments of whole sediment from fjordstations around Spitsbergen at 79°N (Station J, Smeerenburgfjorden),using methods described in Hansen et al. (2000). Thesediment temperature was only 0.5 °C. Isotope fractionationswere determined following the decrease in sulfate concentrationwith time <strong>and</strong> the associated shift in sulfur isotope compositionof dissolved sulfate according to methods described in Habicht<strong>and</strong> Canfield (1997). The isotope fractionation factors (ε) werecalculated using the Rayleigh equation (Brüchert et al., 2001).Isotope fractionations between 9‰ <strong>and</strong> 18‰ were determinedfor surface sediment (0–3 cm depth) (Fig. 5). Sediment from the3–6 cm sediment depth interval, however, yielded a fractionationof 35‰. These results are consistent with the molecular ecologicalanalysis of the community of sulfate-reducing bacteriain the upper 10 cm of sediment at this station, based on 16SrRNA-targeted FISH with oligonucleotide probes (Ravenschlaget al., 2000). Their results indicated the overall dominance ofcomplete-oxidizing bacteria of the nutritionally versatile clusterDesulfosarcina/Desulfococcus spp. (>70%). However, incomplete-oxidizingbacteria, such as Desulfovibrio <strong>and</strong> Desulfotaleaspecies, were relatively more abundant in the uppermostcentimeter, consistent with the lower whole-sediment isotopefractionation. The whole-sediment sulfate reduction rate did notcorrespond to the change in isotope fractionation. These ratesvaried only between 5 <strong>and</strong> 15 nmol cm −3 day −1 , with the higherrates in sediment intervals with higher fractionations.<strong>Sulfur</strong> Isotope Fractionation Associated with CoupledSulfate Reduction <strong>and</strong> Anaerobic Oxidation of MethaneA unique marine environment is that associated with theanaerobic oxidation of methane (Valentine <strong>and</strong> Reeburgh, 2000).There is molecular genetic evidence that this process is catalyzedby a syntrophic association of Archaea <strong>and</strong> sulfate-reducing bacteria(Boetius et al., 2000). Hydrogen <strong>and</strong> formate have been proposedas potential interspecies transfer agents. In this environment,the substrate spectra for the sulfate-reducing bacteria are defined