- Page 2: Sulfur Biogeochemistry—Past and P
- Page 8: Preface viisulfurization on the pre
- Page 11 and 12: 2 V. BrüchertThe resulting sulfur
- Page 13 and 14: 4 V. BrüchertFigure 1. Phylogeneti
- Page 15 and 16: 6 V. Brüchertnot yet been resolved
- Page 17 and 18: 8 V. BrüchertEFFECT OF ELECTRON DO
- Page 19 and 20: 10 V. Brüchertfractionation factor
- Page 21 and 22: 12 V. BrüchertFigure 4. Depth prof
- Page 23 and 24: 14 V. Brüchertto derive isotope fr
- Page 25 and 26: 16 V. BrüchertRudnicki, M.D., Elde
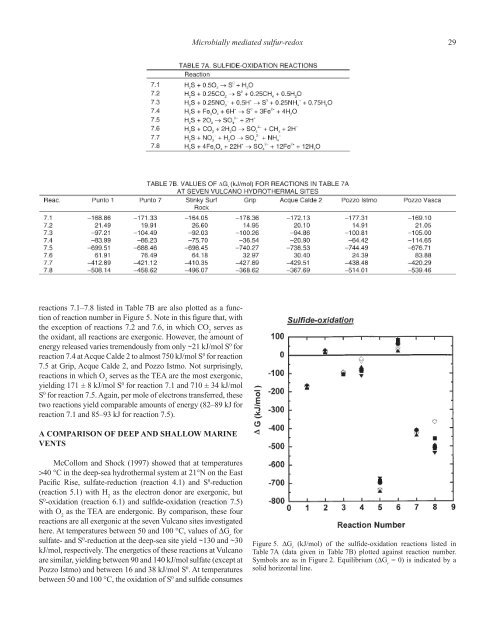
- Page 28 and 29: Microbially mediated sulfur-redox 1
- Page 30 and 31: Microbially mediated sulfur-redox 2
- Page 32 and 33: Microbially mediated sulfur-redox 2
- Page 34 and 35: Microbially mediated sulfur-redox 2
- Page 36 and 37: Microbially mediated sulfur-redox 2
- Page 40 and 41: Microbially mediated sulfur-redox 3
- Page 42 and 43: Microbially mediated sulfur-redox 3
- Page 44 and 45: Geological Society of AmericaSpecia
- Page 46 and 47: Eukaryotes of the Cariaco, Soledad,
- Page 48 and 49: Eukaryotes of the Cariaco, Soledad,
- Page 50 and 51: Eukaryotes of the Cariaco, Soledad,
- Page 52 and 53: Eukaryotes of the Cariaco, Soledad,
- Page 54 and 55: Eukaryotes of the Cariaco, Soledad,
- Page 56: Eukaryotes of the Cariaco, Soledad,
- Page 59 and 60: 50 A. SchippersTABLE 1. DETECTION O
- Page 61 and 62: 52 A. SchippersS 4O 62−+ H 2O →
- Page 63 and 64: 54 A. Schippersthe oxygen molecule
- Page 65 and 66: 56 A. Schippersprohibits biological
- Page 67 and 68: 58 A. SchippersSampleTABLE 4. SULFU
- Page 69 and 70: 60 A. SchippersGeochimica et Cosmoc
- Page 71 and 72: 62 A. SchippersSasaki, K., Tsunekaw
- Page 73 and 74: 64 B.B. Jørgensen and D.C. NelsonI
- Page 75 and 76: 66 B.B. Jørgensen and D.C. NelsonF
- Page 77 and 78: 68 B.B. Jørgensen and D.C. Nelsonq
- Page 79 and 80: 70 B.B. Jørgensen and D.C. Nelsons
- Page 81 and 82: 72 B.B. Jørgensen and D.C. Nelsonp
- Page 83 and 84: 74 B.B. Jørgensen and D.C. NelsonP
- Page 85 and 86: 76 B.B. Jørgensen and D.C. Nelsont
- Page 87 and 88: 78 B.B. Jørgensen and D.C. NelsonF
- Page 89 and 90:
80 B.B. Jørgensen and D.C. NelsonJ
- Page 92 and 93:
Geological Society of AmericaSpecia
- Page 94 and 95:
Formation and degradation of seafl
- Page 96 and 97:
Formation and degradation of seafl
- Page 98 and 99:
Formation and degradation of seafl
- Page 100 and 101:
Formation and degradation of seafl
- Page 102 and 103:
Formation and degradation of seafl
- Page 104 and 105:
Formation and degradation of seafl
- Page 106 and 107:
Geological Society of AmericaSpecia
- Page 108 and 109:
Distribution and fate of sulfur int
- Page 110 and 111:
Distribution and fate of sulfur int
- Page 112 and 113:
Distribution and fate of sulfur int
- Page 114 and 115:
Distribution and fate of sulfur int
- Page 116 and 117:
Distribution and fate of sulfur int
- Page 118 and 119:
Distribution and fate of sulfur int
- Page 120 and 121:
Distribution and fate of sulfur int
- Page 122 and 123:
Distribution and fate of sulfur int
- Page 124 and 125:
Distribution and fate of sulfur int
- Page 126 and 127:
Geological Society of AmericaSpecia
- Page 128 and 129:
- HSO 4SO 2-4H 2 SCO 32-HCO 3-HCO 3
- Page 130 and 131:
Sedimentary pyrite formation 121mar
- Page 132 and 133:
Sedimentary pyrite formation 123The
- Page 134 and 135:
Sedimentary pyrite formation 125Ben
- Page 136 and 137:
Sedimentary pyrite formation 127TAB
- Page 138 and 139:
Sedimentary pyrite formation 129The
- Page 140 and 141:
Sedimentary pyrite formation 131Dek
- Page 142 and 143:
Sedimentary pyrite formation 133Mor
- Page 144 and 145:
Geological Society of AmericaSpecia
- Page 146 and 147:
Recent advances and future research
- Page 148 and 149:
Recent advances and future research
- Page 150 and 151:
Recent advances and future research
- Page 152 and 153:
Recent advances and future research
- Page 154 and 155:
Recent advances and future research
- Page 156 and 157:
Recent advances and future research
- Page 158 and 159:
Recent advances and future research
- Page 160 and 161:
Geological Society of AmericaSpecia
- Page 162 and 163:
Using sulfur isotopes to elucidate
- Page 164 and 165:
Using sulfur isotopes to elucidate
- Page 166 and 167:
Using sulfur isotopes to elucidate
- Page 168 and 169:
Using sulfur isotopes to elucidate
- Page 170 and 171:
Geological Society of AmericaSpecia
- Page 172 and 173:
Sites of anomalous organic reminera
- Page 174 and 175:
Sites of anomalous organic reminera
- Page 176 and 177:
Sites of anomalous organic reminera
- Page 178 and 179:
Sites of anomalous organic reminera
- Page 180 and 181:
Sites of anomalous organic reminera
- Page 182 and 183:
Sites of anomalous organic reminera
- Page 184 and 185:
Sites of anomalous organic reminera
- Page 186 and 187:
Geological Society of AmericaSpecia
- Page 188 and 189:
Sulfur isotope composition of carbo
- Page 190 and 191:
Sulfur isotope composition of carbo
- Page 192 and 193:
Sulfur isotope composition of carbo
- Page 194 and 195:
Sulfur isotope composition of carbo
- Page 196 and 197:
Sulfur isotope composition of carbo
- Page 198 and 199:
Sulfur isotope composition of carbo
- Page 200 and 201:
Sulfur isotope composition of carbo
- Page 202 and 203:
Sulfur isotope composition of carbo
- Page 204 and 205:
Geological Society of AmericaSpecia
- Page 206 and 207:
4 Ga of seawater evolution 197brine
- Page 208 and 209:
4 Ga of seawater evolution 199Figur
- Page 210 and 211:
4 Ga of seawater evolution 201data
- Page 212 and 213:
4 Ga of seawater evolution 203notab
- Page 214:
4 Ga of seawater evolution 205et Co