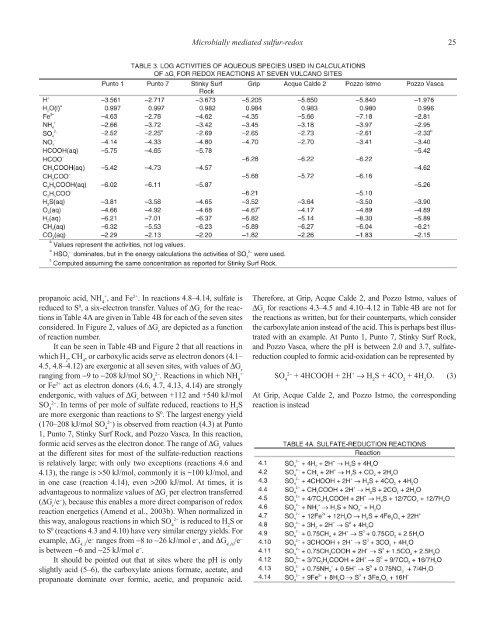
Microbially mediated sulfur-redox 25propanoic acid, NH 4+, <strong>and</strong> Fe 2+ . In reactions 4.8–4.14, sulfate isreduced to S 0 , a six-electron transfer. Values of ∆G rfor the reactionsin Table 4A are given in Table 4B for each of the seven sitesconsidered. In Figure 2, values of ∆G rare depicted as a functionof reaction number.It can be seen in Table 4B <strong>and</strong> Figure 2 that all reactions inwhich H 2, CH 4, or carboxylic acids serve as electron donors (4.1–4.5, 4.8–4.12) are exergonic at all seven sites, with values of ∆G rranging from −9 to −208 kJ/mol SO 42−. Reactions in which NH 4+or Fe 2+ act as electron donors (4.6, 4.7, 4.13, 4.14) are stronglyendergonic, with values of ∆G rbetween +112 <strong>and</strong> +540 kJ/molSO 42−. In terms of per mole of sulfate reduced, reactions to H 2Sare more exergonic than reactions to S 0 . The largest energy yield(170–208 kJ/mol SO 42−) is observed from reaction (4.3) at Punto1, Punto 7, Stinky Surf Rock, <strong>and</strong> Pozzo Vasca. In this reaction,formic acid serves as the electron donor. The range of ∆G rvaluesat the different sites for most of the sulfate-reduction reactionsis relatively large; with only two exceptions (reactions 4.6 <strong>and</strong>4.13), the range is >50 kJ/mol, commonly it is ~100 kJ/mol, <strong>and</strong>in one case (reaction 4.14), even >200 kJ/mol. At times, it isadvantageous to normalize values of ∆G rper electron transferred(∆G r/e − ), because this enables a more direct comparison of redoxreaction energetics (Amend et al., 2003b). When normalized inthis way, analogous reactions in which SO 42−is reduced to H 2S orto S 0 (reactions 4.3 <strong>and</strong> 4.10) have very similar energy yields. Forexample, ∆G 4.3/e − ranges from −8 to −26 kJ/mol e − , <strong>and</strong> ∆G 4.10/e −is between −6 <strong>and</strong> −25 kJ/mol e − .It should be pointed out that at sites where the pH is onlyslightly acid (5–6), the carboxylate anions formate, acetate, <strong>and</strong>propanoate dominate over formic, acetic, <strong>and</strong> propanoic acid.Therefore, at Grip, Acque Calde 2, <strong>and</strong> Pozzo Istmo, values of∆G rfor reactions 4.3–4.5 <strong>and</strong> 4.10–4.12 in Table 4B are not forthe reactions as written, but for their counterparts, which considerthe carboxylate anion instead of the acid. This is perhaps best illustratedwith an example. At Punto 1, Punto 7, Stinky Surf Rock,<strong>and</strong> Pozzo Vasca, where the pH is between 2.0 <strong>and</strong> 3.7, sulfatereductioncoupled to formic acid-oxidation can be represented bySO 42−+ 4HCOOH + 2H + → H 2S + 4CO 2+ 4H 2O. (3)At Grip, Acque Calde 2, <strong>and</strong> Pozzo Istmo, the correspondingreaction is instead
26 J.P. Amend, K.L. Rogers, <strong>and</strong> D.R. Meyer-DombardSO 42−+ 4HCOO − + 6H + → H 2S + 4CO 2+ 4H 2O. (4)By the same argument, S 0 -reduction reactions (see below) atGrip, Acque Calde 2, <strong>and</strong> Pozzo Istmo consider the carboxylateanion, but at Punto 1, Punto 7, Stinky Surf Rock, <strong>and</strong> PozzoVasca, they consider the protonated form. In each case, thenumber of protons is adjusted accordingly on the left h<strong>and</strong> sideof the reactions.It should be pointed out that reactions 4.3–4.5 <strong>and</strong> 4.10–4.12 are less energy-yielding (or less energy-consuming) atGrip, Acque Calde 2, <strong>and</strong> Pozzo Istmo than at Punto 1, Punto7, Stinky Surf Rock, <strong>and</strong> Pozzo Vasca. In other words, the chemoorganotrophicreactions considered here are thermodynamicallymore favorable at strongly acid sites than at weakly acidsites. If we again consider reaction 4.3 as an example, we seein Table 4B <strong>and</strong> Figure 2 that values of ∆G 4.3are ~110 kJ/molless negative at Grip, Acque Calde 2, <strong>and</strong> Pozzo Istmo than atPunto 1, Punto 7, Stinky Surf Rock, <strong>and</strong> Pozzo Vasca. Althoughthese thermodynamic calculations show that more energy isreleased from sulfate-reduction at lower pH, it has not yet beendemonstrated whether microbial sulfate-reduction actuallyoccurs under conditions of low pH <strong>and</strong> high temperature. Sulfate-reductionhas been measured at low pH (Kühl et al., 1998),but little is known about the effect of pH on the compositionof sulfate-reducing microbial populations <strong>and</strong> what species ofsulfate reducers are active under acidic conditions. In a recentstudy (Küsel et al., 2001), a sulfate-reducing bacterium with apH growth optimum of 5.5 was isolated from an acidic environment.This bacterium continued to reduce sulfate at a pHvalue as low as 4.9. Although this finding suggests that sulfatereducers may be present in low pH habitats at mesophilic temperatures,nothing is known about the presence <strong>and</strong> identity ofsulfate reducers in low-pH/high-temperature environments. Inaddition, no sulfate reducer has been described to date that canbe considered a true acidophile (i.e., with a pH optimum
- Page 2: Sulfur Biogeochemistry—Past and P
- Page 8: Preface viisulfurization on the pre
- Page 11 and 12: 2 V. BrüchertThe resulting sulfur
- Page 13 and 14: 4 V. BrüchertFigure 1. Phylogeneti
- Page 15 and 16: 6 V. Brüchertnot yet been resolved
- Page 17 and 18: 8 V. BrüchertEFFECT OF ELECTRON DO
- Page 19 and 20: 10 V. Brüchertfractionation factor
- Page 21 and 22: 12 V. BrüchertFigure 4. Depth prof
- Page 23 and 24: 14 V. Brüchertto derive isotope fr
- Page 25 and 26: 16 V. BrüchertRudnicki, M.D., Elde
- Page 28 and 29: Microbially mediated sulfur-redox 1
- Page 30 and 31: Microbially mediated sulfur-redox 2
- Page 32 and 33: Microbially mediated sulfur-redox 2
- Page 36 and 37: Microbially mediated sulfur-redox 2
- Page 38 and 39: Microbially mediated sulfur-redox 2
- Page 40 and 41: Microbially mediated sulfur-redox 3
- Page 42 and 43: Microbially mediated sulfur-redox 3
- Page 44 and 45: Geological Society of AmericaSpecia
- Page 46 and 47: Eukaryotes of the Cariaco, Soledad,
- Page 48 and 49: Eukaryotes of the Cariaco, Soledad,
- Page 50 and 51: Eukaryotes of the Cariaco, Soledad,
- Page 52 and 53: Eukaryotes of the Cariaco, Soledad,
- Page 54 and 55: Eukaryotes of the Cariaco, Soledad,
- Page 56: Eukaryotes of the Cariaco, Soledad,
- Page 59 and 60: 50 A. SchippersTABLE 1. DETECTION O
- Page 61 and 62: 52 A. SchippersS 4O 62−+ H 2O →
- Page 63 and 64: 54 A. Schippersthe oxygen molecule
- Page 65 and 66: 56 A. Schippersprohibits biological
- Page 67 and 68: 58 A. SchippersSampleTABLE 4. SULFU
- Page 69 and 70: 60 A. SchippersGeochimica et Cosmoc
- Page 71 and 72: 62 A. SchippersSasaki, K., Tsunekaw
- Page 73 and 74: 64 B.B. Jørgensen and D.C. NelsonI
- Page 75 and 76: 66 B.B. Jørgensen and D.C. NelsonF
- Page 77 and 78: 68 B.B. Jørgensen and D.C. Nelsonq
- Page 79 and 80: 70 B.B. Jørgensen and D.C. Nelsons
- Page 81 and 82: 72 B.B. Jørgensen and D.C. Nelsonp
- Page 83 and 84: 74 B.B. Jørgensen and D.C. NelsonP
- Page 85 and 86:
76 B.B. Jørgensen and D.C. Nelsont
- Page 87 and 88:
78 B.B. Jørgensen and D.C. NelsonF
- Page 89 and 90:
80 B.B. Jørgensen and D.C. NelsonJ
- Page 92 and 93:
Geological Society of AmericaSpecia
- Page 94 and 95:
Formation and degradation of seafl
- Page 96 and 97:
Formation and degradation of seafl
- Page 98 and 99:
Formation and degradation of seafl
- Page 100 and 101:
Formation and degradation of seafl
- Page 102 and 103:
Formation and degradation of seafl
- Page 104 and 105:
Formation and degradation of seafl
- Page 106 and 107:
Geological Society of AmericaSpecia
- Page 108 and 109:
Distribution and fate of sulfur int
- Page 110 and 111:
Distribution and fate of sulfur int
- Page 112 and 113:
Distribution and fate of sulfur int
- Page 114 and 115:
Distribution and fate of sulfur int
- Page 116 and 117:
Distribution and fate of sulfur int
- Page 118 and 119:
Distribution and fate of sulfur int
- Page 120 and 121:
Distribution and fate of sulfur int
- Page 122 and 123:
Distribution and fate of sulfur int
- Page 124 and 125:
Distribution and fate of sulfur int
- Page 126 and 127:
Geological Society of AmericaSpecia
- Page 128 and 129:
- HSO 4SO 2-4H 2 SCO 32-HCO 3-HCO 3
- Page 130 and 131:
Sedimentary pyrite formation 121mar
- Page 132 and 133:
Sedimentary pyrite formation 123The
- Page 134 and 135:
Sedimentary pyrite formation 125Ben
- Page 136 and 137:
Sedimentary pyrite formation 127TAB
- Page 138 and 139:
Sedimentary pyrite formation 129The
- Page 140 and 141:
Sedimentary pyrite formation 131Dek
- Page 142 and 143:
Sedimentary pyrite formation 133Mor
- Page 144 and 145:
Geological Society of AmericaSpecia
- Page 146 and 147:
Recent advances and future research
- Page 148 and 149:
Recent advances and future research
- Page 150 and 151:
Recent advances and future research
- Page 152 and 153:
Recent advances and future research
- Page 154 and 155:
Recent advances and future research
- Page 156 and 157:
Recent advances and future research
- Page 158 and 159:
Recent advances and future research
- Page 160 and 161:
Geological Society of AmericaSpecia
- Page 162 and 163:
Using sulfur isotopes to elucidate
- Page 164 and 165:
Using sulfur isotopes to elucidate
- Page 166 and 167:
Using sulfur isotopes to elucidate
- Page 168 and 169:
Using sulfur isotopes to elucidate
- Page 170 and 171:
Geological Society of AmericaSpecia
- Page 172 and 173:
Sites of anomalous organic reminera
- Page 174 and 175:
Sites of anomalous organic reminera
- Page 176 and 177:
Sites of anomalous organic reminera
- Page 178 and 179:
Sites of anomalous organic reminera
- Page 180 and 181:
Sites of anomalous organic reminera
- Page 182 and 183:
Sites of anomalous organic reminera
- Page 184 and 185:
Sites of anomalous organic reminera
- Page 186 and 187:
Geological Society of AmericaSpecia
- Page 188 and 189:
Sulfur isotope composition of carbo
- Page 190 and 191:
Sulfur isotope composition of carbo
- Page 192 and 193:
Sulfur isotope composition of carbo
- Page 194 and 195:
Sulfur isotope composition of carbo
- Page 196 and 197:
Sulfur isotope composition of carbo
- Page 198 and 199:
Sulfur isotope composition of carbo
- Page 200 and 201:
Sulfur isotope composition of carbo
- Page 202 and 203:
Sulfur isotope composition of carbo
- Page 204 and 205:
Geological Society of AmericaSpecia
- Page 206 and 207:
4 Ga of seawater evolution 197brine
- Page 208 and 209:
4 Ga of seawater evolution 199Figur
- Page 210 and 211:
4 Ga of seawater evolution 201data
- Page 212 and 213:
4 Ga of seawater evolution 203notab
- Page 214:
4 Ga of seawater evolution 205et Co