Lecture 28: Heteronuclear Diatomic Molecules The material ... - Cobalt
Lecture 28: Heteronuclear Diatomic Molecules The material ... - Cobalt
Lecture 28: Heteronuclear Diatomic Molecules The material ... - Cobalt
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
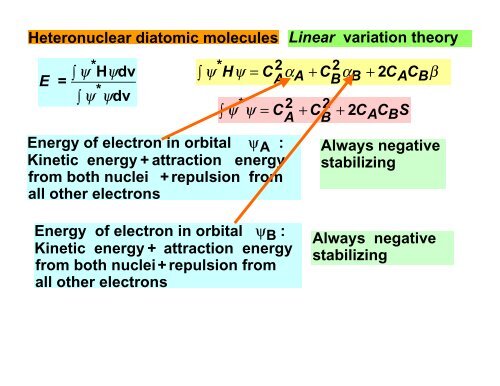
<strong>Heteronuclear</strong> diatomic molecules Linear variation theoryE =*∫ ψ Hψdvψψ*∫ dvψ*∫ H ψ = C2α + C2α + 2 C C βA A B B A B∫ ψψ* = C2+ C2+ 2C C SA B A BEnergy of electron in orbital ψ A : Always negativeKinetic energy + attraction energy stabilizingfrom both nuclei + repulsion fromall other electronsEnergy of electron in orbital ψ B :Kinetic energy + attraction energyfrom both nuclei+ repulsion fromall other electronsAlways negativestabilizing